First-in-Human Clinical-Grade Porcine Kidney Xenotransplant
1University of Alabama at Birmingham, Birmingham, AL, 2Deakin University, Geelong, Australia, 3Revivicor, Incorporated, Blacksburg, VA, 4University of Alabama at Birmingham, Irondale, AL
Meeting: 2022 American Transplant Congress
Abstract number: 83
Keywords: Brain death, Kidney transplantation, Pig, Xenoreactive antibodies
Topic: Basic Science » Basic Science » 13 - Xenotransplantation
Session Information
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:30pm-4:40pm
Presentation Time: 4:30pm-4:40pm
Location: Hynes Room 302
*Purpose: Proof-of-concept xenotransplant work has been performed in pig to non-human primates (NHP), but it is unclear if outcomes can be recapitulated in humans. While brain death pathophysiology may create a hostile environment and limit functional assessment, a human brain death model would permit safety/feasibility assessments (risk of hyperacute rejection, surgical complications, viral transmission). We present results of the first-in-human, clinical-grade xenotransplant in a brain-death model.
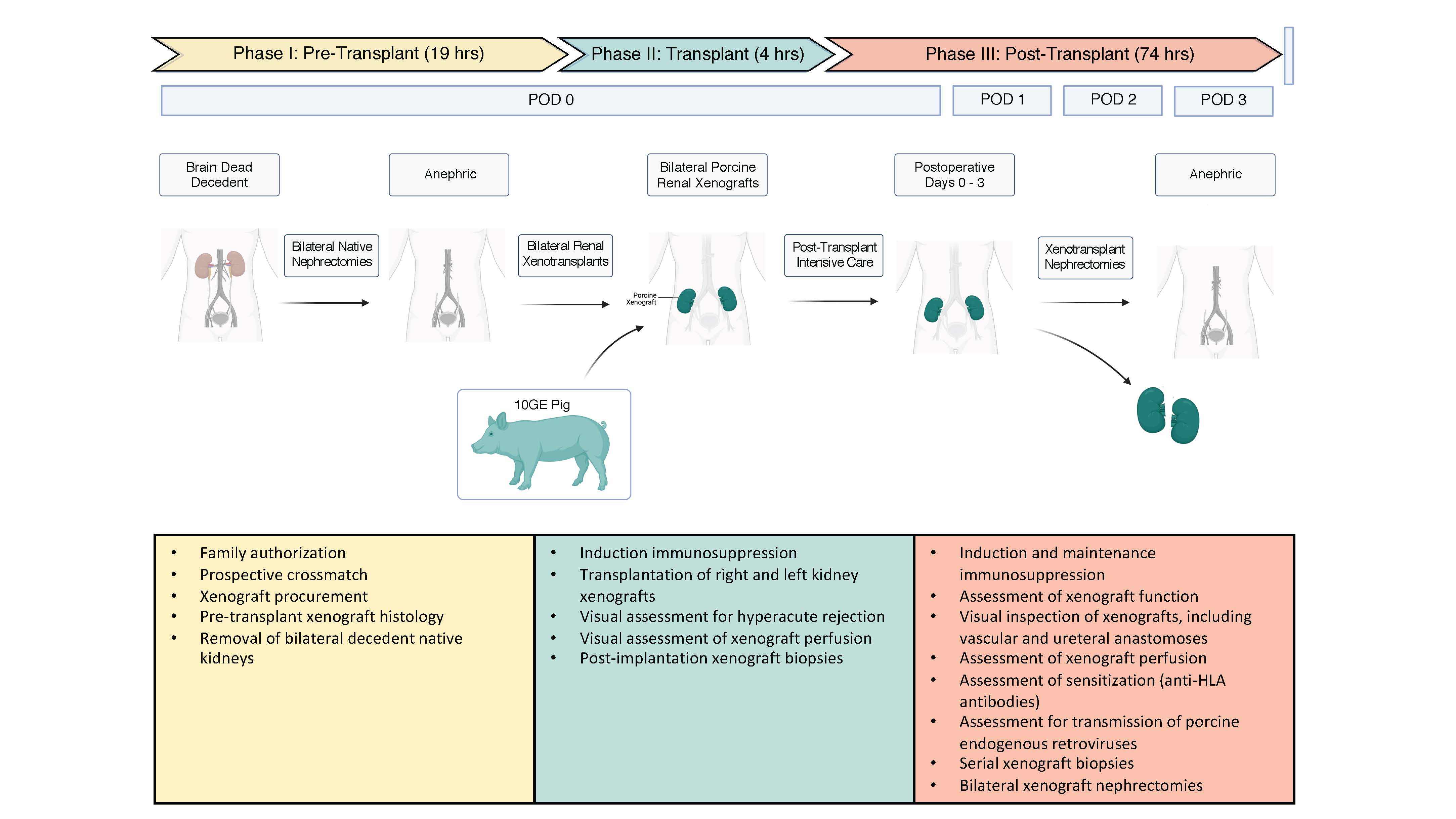
*Methods: Next-of-kin to a 57-year-old brain dead male consented to participate. After a negative xeno-specific prospective crossmatch, the decedent underwent bilateral nephrectomy and dual kidney xenotransplant from a 10-gene modified pig and standard immunosuppression. We took serial blood, urine and tissue samples for 74 hours before study termination.
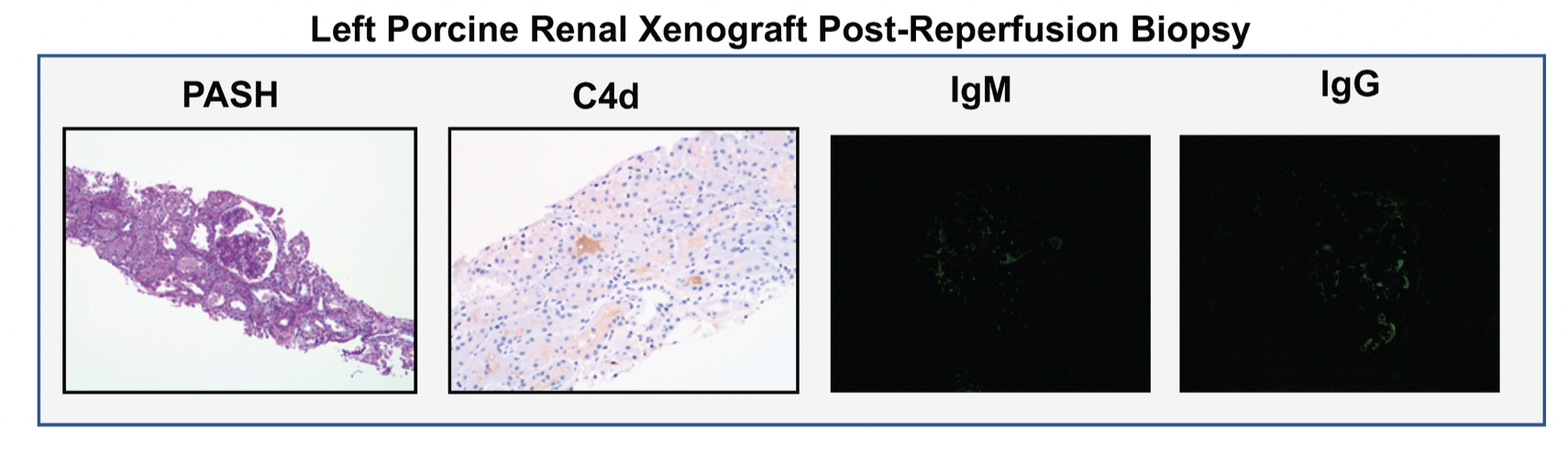
*Results: Both kidneys reperfused with excellent color and turgor–maintained throughout the study–without hyperacute rejection. There was no anastomotic bleeding or parenchymal disruption despite human arterial pressure. Day 1 biopsies were consistent with TMA. At day 3 there was progressive tubular injury with extensive ATN, but TMA was not observed. C4d, IgM, IgG, IgA, C1q, and C3 were negative at both time points. Wedge biopsies at study end showed no cortical necrosis or interstitial hemorrhage and ATN was now patchy and glomerular capillary congestion no longer diffuse. Both kidneys made urine, but renal function was not demonstrated. Decedent blood tested for porcine viruses remained negative.
*Conclusions: We addressed critical safety and feasibility issues in xenotransplant in a novel pre-clinical human model. This model identified areas where additional understanding is needed, though they are an important adjunct to data generated in NHP models and motivate clinical trials in living humans.
To cite this abstract in AMA style:
Locke JE, Orandi B, Kumar V, Houp J, Anderson D, Hauptfeld V, Martin D, Killian C, Macedon S, Budd N, Stegner K, Dandro A, Kokkinaki M, Kuravi K, Reed R, Fatima H, Baker G, Perry J, Bryant E, Cheung M, Erman E, Kraebber K, Gamblin T, Guy L, Ayares D, Porrett P. First-in-Human Clinical-Grade Porcine Kidney Xenotransplant [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/first-in-human-clinical-grade-porcine-kidney-xenotransplant/. Accessed February 26, 2026.« Back to 2022 American Transplant Congress