Fifteen Year Outcomes in the Astellas Double Blind Randomized Controlled Trial (RCT) Comparing Early Corticosteroid Withdrawal (CSWD) and Long-Term Corticosteroid Therapy Continuation (CCS) in Kidney Transplantation
1Transplant Surgery, University of Cincinnati College of Medicine, Cincinnati, OH, 2Providence Health Care, Vancouver, BC, Canada, 3UNOS, Richmond, VA, 4Transplant Genomics Inc. and Comprehensive Transplant Center, Northwestern University, Evanston, IL, 5University of Cincinnati College of Medicine, Cincinnati, OH, 6University of British Columbia, Vancouver, BC, Canada, 7Yale University, New Haven, CT
Meeting: 2019 American Transplant Congress
Abstract number: 202
Keywords: Graft function, Graft survival, Kidney transplantation, Multicenter studies
Session Information
Session Time: 8:30am-9:15am
 Presentation Time: 8:45am-9:00am
Presentation Time: 8:45am-9:00am
Location: Veterans Auditorium
*Purpose: To evaluate long-term outcomes among participants in the original multi-center RCT initiated in 1999 (Woodle et al, Annals of Surgery, 2008), and to demonstrate the use and limitations of UNOS/OPTN data in ascertaining long-term outcomes in RCT participants.
*Methods: Astellas provided the original study data file containing identifying data for participants. UNOS used a multi-step process to link participants to UNOS/OPTN data. A de-identified data set was produced and the original study data was returned to Astellas. Both intention to treat (ITT) and per protocol (PPR) analyses were performed: 70 CSWD and 73 CCS patients had discontinued the trial assigned treatment protocol by five years after the date of transplantation. We were unable to determine protocol discontinuation beyond five years because of absent data regarding maintenance immunosuppressant use.
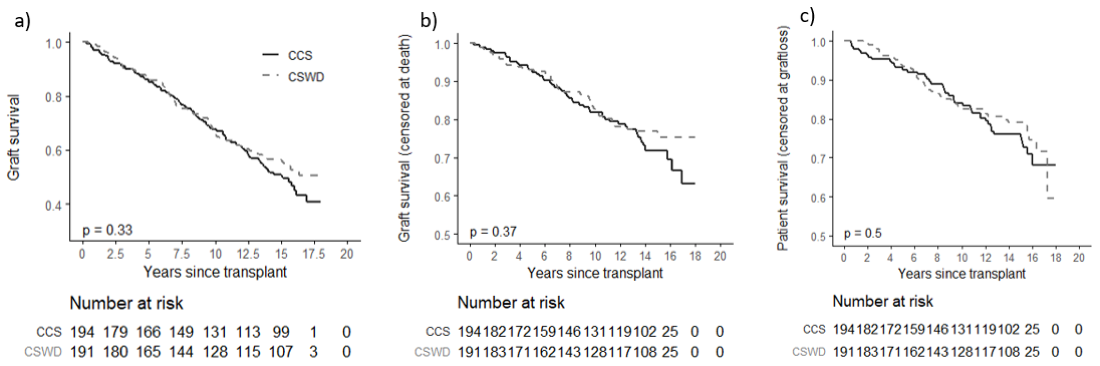
*Results: Follow up to fifteen years, and outcomes could be determined in 385/386 study participants in UNOS files including all 196 of the patients randomized to CSWD at 7 days after transplantation, and 191/192 of the patients randomized to CCS. The figure shows that time to allograft failure from any cause including death (a), death censored allograft failure (b), and death with allograft function (c) were similar in patients randomized to CSWD or CCS (results were unchanged in PPR analyses). After 15 years, the MDRD estimated GFR was 55.7± 24.0 ml/min/1.73m2 in CSWD and 55.3 ± 27.2 ml/min/1.73m2 in the CCS groups with similar results in PPR analyses.
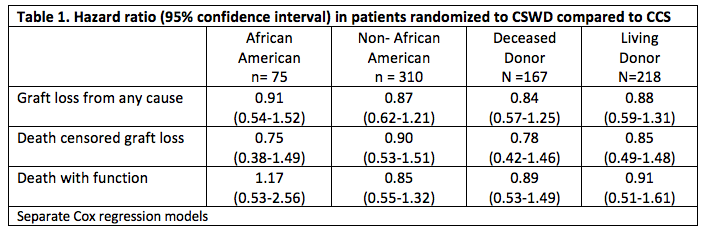
The results were consistent in stratified analyses among African American (AA) and non-African American participants, and among living and deceased donor recipients (Table 1).
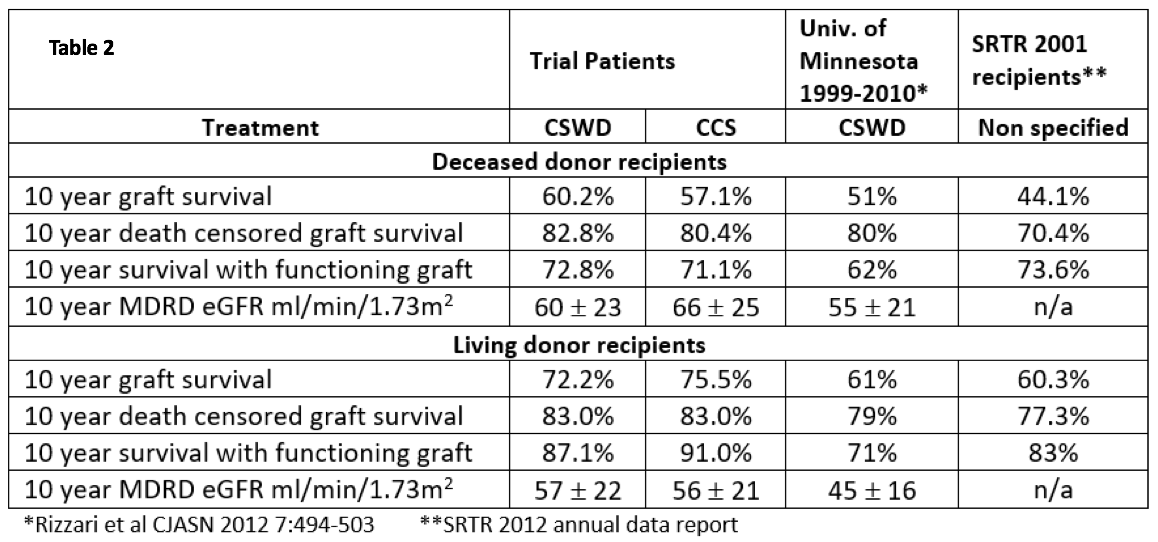
Table 2 shows 10 year outcomes stratified by donor source compared to single center data in patients treated with CSWD and the University of Minnesota (Rizzari et al CJASN 2012), and contemporary national data (2002 SRTR ADR). Irrespective of treatment group, outcomes in trial participants were generally superior compared to single center or national outcomes.
*Conclusions: Conclusions: Patients randomized to treatment with early CSWD or CCS had near identical outcomes after 15 years of follow-up with consistent results in AA and non-AA participants, and among living and deceased donor transplant recipients. The UNOS registry can be successfully utilized to obtain long-term RCT outcomes and this capacity should be expanded to promote future trials in transplantation.
To cite this abstract in AMA style:
Woodle E, Clark S, Stewart D, First M, Alloway R, Gill J, Group SplitRock. Fifteen Year Outcomes in the Astellas Double Blind Randomized Controlled Trial (RCT) Comparing Early Corticosteroid Withdrawal (CSWD) and Long-Term Corticosteroid Therapy Continuation (CCS) in Kidney Transplantation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/fifteen-year-outcomes-in-the-astellas-double-blind-randomized-controlled-trial-rct-comparing-early-corticosteroid-withdrawal-cswd-and-long-term-corticosteroid-therapy-continuation-ccs-in-kidney/. Accessed March 2, 2026.« Back to 2019 American Transplant Congress