Explorative Analysis of ZEUS After 5 Years: Histological Assessment from Biopsy Analyses
1ZEUS Study Group, Germany
2Novartis, Pharma, Germany
3ZEUS Study Group, Switzerland.
Meeting: 2015 American Transplant Congress
Abstract number: D119
Keywords: Biopsy, Immunosuppression, Kidney transplantation, Multicenter studies
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Drug Minimization
Session Type: Poster Session
Date: Tuesday, May 5, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Background: Analysis of pathologists' assessments and histological data allow for deeper insight on patient outcome together with investigator's final clinical diagnoses. Here we present 5 year data from de novo transplant recipients after conversion to an everolimus (EVR) based regimen and withdrawal of calcineurin inhibitor therapy.
Methods: Analysis of histological and pathologists assessments from the prospective, open-label, controlled, multi-center study ZEUS. 300 renal transplant (Tx) patients were randomized at month (Mo)4.5 post Tx to either receive EVR plus enteric coated-mycophenolate sodium (EC-MPS ) (n=154) or cyclosporine (CsA) plus EC-MPS regimen (n=146). After 12 Mo interventional core study observational follow-up (FU) on pts safety and efficacy was performed until Mo60 post Tx.
Results: Total number (nr) of biopsies performed and mean nr of biopsies per patient are overall similar in both groups until Mo60. Nr of pts with at least one rejection (as per final clinical diagnosis) was slightly higher in CNI group vs EVR group. Nr of pts with BPAR was higher in the EVR group especially due to mild, early acute rejections (mostly BANFF IA and IB). Nr of pts with histological evidence of chronic/sclerosing allograft nephropathy was similar in both groups, C4D staining positivity was found slightly higher in EVR group, however, pts with evidence of antibody mediated rejection was higher in CNI group as well as CNI induced toxicity. 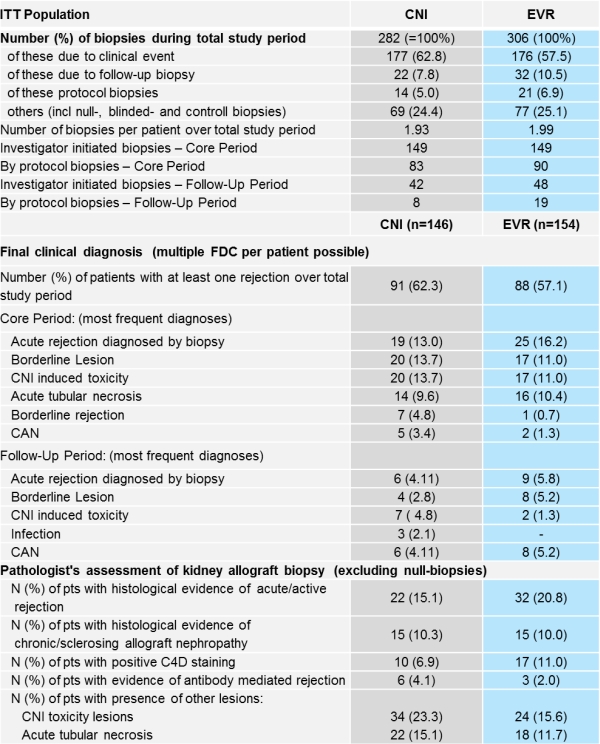
Conclusions: Data from histological assessments and reported final clinical outcomes show that an EVR-based regimen with early elimination of CNI therapy is as safe and efficacious as standard CNI therapy offering the opportunity to reduce CNI-induced toxicities on the allograft.
To cite this abstract in AMA style:
Eisenberger U, Budde K, Witzke O, Lehner F, Sommerer C, Wuethrich R, Reinke P, Muehlfeld A, Heller K, Stahl R, Wolters H, Hauser I, Porstner M, Arns W. Explorative Analysis of ZEUS After 5 Years: Histological Assessment from Biopsy Analyses [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/explorative-analysis-of-zeus-after-5-years-histological-assessment-from-biopsy-analyses/. Accessed March 5, 2026.« Back to 2015 American Transplant Congress
