Evaluation of Leukopenia and Neutropenia Incidence, Treatment, and Clinical Response in Kidney and Liver Transplant Recipients
Hartford Hospital, Hartford, CT
Meeting: 2020 American Transplant Congress
Abstract number: B-131
Keywords: Adverse effects, Immunosuppression, Kidney transplantation, Liver transplantation
Session Information
Session Name: Poster Session B: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: The purpose is to evaluate incidence, recurrence, therapy modifications, and complications of leukopenia and neutropenia (LN) in kidney (KTR) and liver transplant (LTR) recipients.
*Methods: KTR and LTR transplanted at Hartford Hospital from 8/20/2016-6/3/2018 were retrospectively evaluated. Patients were excluded if they were <18 years old, died on day of transplant, or had a multiorgan transplant. Outcomes were evaluated from transplant to 1 year post-transplant. LN was defined as a white blood cell count ≤ 2500/µl or an absolute neutrophil count ≤ 1000/µl. Differences between KTR and LTR were evaluated with chi square and Student’s t-tests at α=0.05.
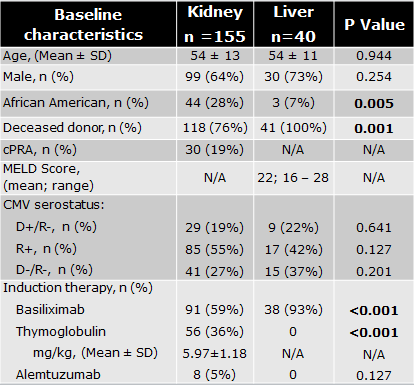
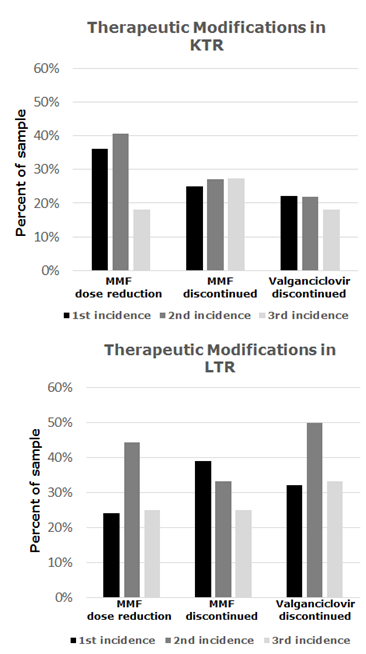
*Results: 196 records were evaluated; 155 KTR and 40 LTR (Table 1). More LTR developed LN compared to KTR (66% vs. 47%, p=0.027). Subsequently, 30% and 29% of KTR and LTR developed a 2nd recurrence (p=0.959), and 13% and 12% developed a 3rd recurrence (p=0.904). 12.7% and 11.1% of KTR and LTR were hospitalized during their 1st incidence due to LN with 4.3% and 8.3%, and 14.3% and 40% at 2nd and 3rd incidences, respectively. In KTR, there was a trend towards more rejection in those who developed LN compared to those who did not (15.3% vs. 6.0%; p=0.059), which was not seen in LTR (14.8% vs. 21.4%; p=0.673). There was no significant difference in mortality rate for KTR and LTR who developed LN. Mycophenolate dose changes and valganciclovir discontinuation were common adjustments during all three incidences in both cohorts (Figure 1). 7% of KTR and no LTR received granulocyte-colony stimulating factor (GCSF) therapy.
*Conclusions: Although LN is a well-documented complication after transplantation, the incidence in this cohort was greater than in previous studies. This cohort also experienced a high recurrence rate, which may be due to restarting LN-inducing medications following recovery. Additionally, KTR who developed LN experienced a high rate of rejection despite minimal use of GCSF, suggesting rejection following LN is likely due to factors other than GCSF utilization. Optimizing valganciclovir duration of therapy and liberalizing the use of GCSF rather than discontinuing mycophenolate may reduce the risk of recurrence of LN and subsequent rejection in future cohorts.
To cite this abstract in AMA style:
Kutzler H, Webb T, Cecere R, O'Sullivan D, Sheiner P. Evaluation of Leukopenia and Neutropenia Incidence, Treatment, and Clinical Response in Kidney and Liver Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-leukopenia-and-neutropenia-incidence-treatment-and-clinical-response-in-kidney-and-liver-transplant-recipients/. Accessed July 18, 2025.« Back to 2020 American Transplant Congress