Evaluation of Conversion from Immediate Release (IR) Tacrolimus to LCP-Tacrolimus (Envarsus XR®, LCP) in Heart Transplant Recipients
1University of Illinois at Chicago, Chicago, IL, 2Loyola University Medical Center, Maywood, IL
Meeting: 2022 American Transplant Congress
Abstract number: 1143
Keywords: Adverse effects, Efficacy, Heart/lung transplantation, Immunosuppression
Topic: Clinical Science » Heart » 63 - Heart and VADs: All Topics
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The purpose of this study was to assess outcomes in heart transplant recipients who were converted from IR tacrolimus to LCPT. Limited data exists assessing safety and efficacy of conversion to the LCPT formulation in heart transplant recipients.
*Methods: This retrospective chart review included patients from a quaternary care heart transplant center who were converted from IR tacrolimus to LCPT and maintained on LCPT for at least 3 months. Patients were screened for eligibility and excluded if LCPT was started de novo, patients were transitioned from a medication other than IR tacrolimus, or patients were on LCPT for a period of less than 3 months. The purpose of this study was to identify potential investigational targets for a future prospective study, particularly surrounding renal, metabolic, and adverse drug effect outcomes in heart transplant recipients.
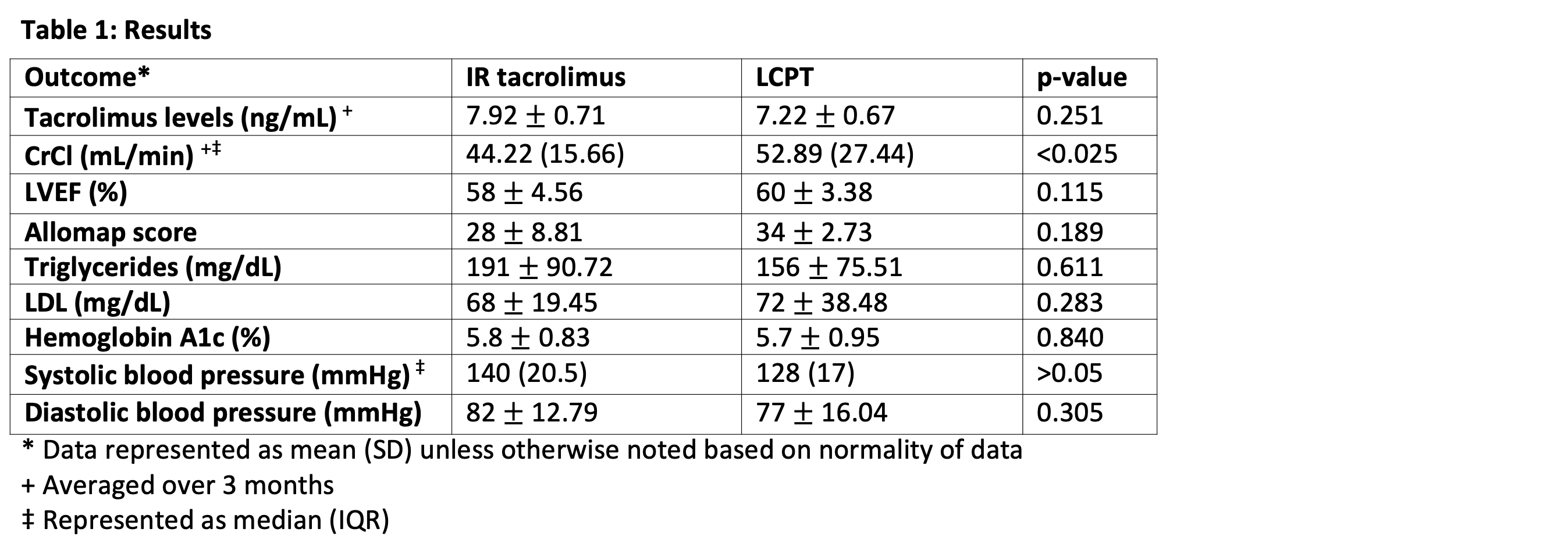
*Results: Ten patients met inclusion criteria and were included in statistical analysis. Patients had significant improvements in creatinine clearance (CrCl) after conversion to LCPT compared to CrCl on IR tacrolimus at 3 months (52.98 vs. 44.22 mL/min, p <0.025). No differences were found in left ventricular ejection fraction (LVEF) or Allomap scores. Metabolic profiles and blood pressure readings remained stable after conversion to LCPT (Table 1). Conversion to LCPT was well tolerated by all patients with no change in the incidence of headache, 1 case of tremor resolution, and 1 case of new tremor.
*Conclusions: Patients on LCPT had significant improvements in renal function when converted from IR tacrolimus without differences in LVEF, Allomap scores, or metabolic profile. Additional prospective studies are needed to confirm the impact on outcomes in patients who are converted from IR tacrolimus to LCPT.
To cite this abstract in AMA style:
Foster E, Iaria A, Negrelli J, Fine M, Yu M, Liebo M. Evaluation of Conversion from Immediate Release (IR) Tacrolimus to LCP-Tacrolimus (Envarsus XR®, LCP) in Heart Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-conversion-from-immediate-release-ir-tacrolimus-to-lcp-tacrolimus-envarsus-xr-lcp-in-heart-transplant-recipients/. Accessed February 27, 2026.« Back to 2022 American Transplant Congress