Enabled PRA and Precision PRA: Alternatives to cPRA as Desensitization Trial Endpoints.
U of Cincinnati, Cincinnati
Mayo Clinic Scottsdale, Scottsdale.
Meeting: 2016 American Transplant Congress
Abstract number: A101
Keywords: Antibodies, Highly-sensitized, HLA antibodies, Immunoglobulins (Ig)
Session Information
Session Name: Poster Session A: Kidney Desensitization
Session Type: Poster Session
Date: Saturday, June 11, 2016
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Halls C&D
Transplant (Transplant) rate is an optimal endpoint in desensitization (DS) trials in HLA sensitized Txp candidates, however, for waitlisted Txp candidates, wait time heterogeneity compromises its integrity. Wait list DS trials have largely been conducted without a defined primary endpoint, using PRA as an efficacy measure. cPRA is an insensitive endpoint in HLA sensitized patients (pts) (eg, those with 100% cPRA) as removal of 50% or more of HLA specificities often does not reduce cPRA. Therefore, cPRA alternatives (enabled PRA (ePRA), precision PRA (pcPRA), reciprocal pcPRA (rpcPRA,defined as 1-rpcPRAd) provides transplantation odds) were developed and assessed.
METHODS
HLA specificities were determined by HLA single antigen bead (SAB) assay. PRA values were determined from pre and post treatment data from a published DS trial using three cutoffs (1500, 4000, and 8000 MFI). ePRA was calculated using HLA specificities that were reduced below the cutoff value by DS. pcPRA was calculated to 10 decimal places using a calculator developed by the author(MJP). Virtual DS was performed by serial elimination of HLA specificities (beginning with highest MFI Ab) with repeated PRA calculations.
RESULTS
Virtual desensitization revealed cPRA insensitivity, whereas [Delta]rpcPRA demonstrated linear reductions with removal of individual HLA specificities. 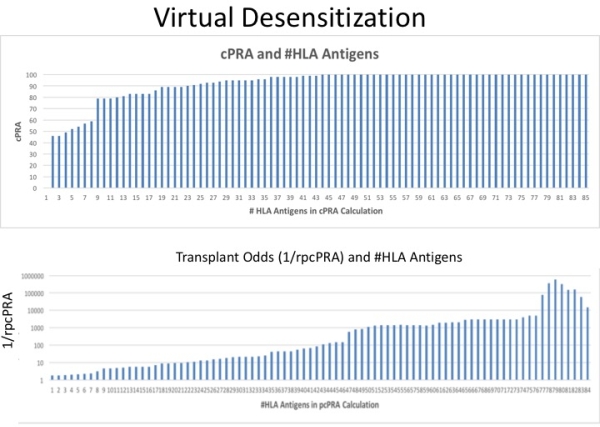 Using DS trial data,the proportion of pts meeting proposed endpoints were: 1) [Delta]cPRA1500 >25%- 15.8% of pts, 2) ePRA1500 >25%- 78.9% of pts, 3) [Delta]rpcPRA > 2 fold- 73.7% of pts. Correlative analysis at multiple MFI cutoffs (1500, 4000, and 8000MFI) of [Delta]cPRA,ePRA, and [Delta]rpcPRA using DS trial data revealed strong correlation between ePRA and [Delta]rpcPRA (r=0.817)
Using DS trial data,the proportion of pts meeting proposed endpoints were: 1) [Delta]cPRA1500 >25%- 15.8% of pts, 2) ePRA1500 >25%- 78.9% of pts, 3) [Delta]rpcPRA > 2 fold- 73.7% of pts. Correlative analysis at multiple MFI cutoffs (1500, 4000, and 8000MFI) of [Delta]cPRA,ePRA, and [Delta]rpcPRA using DS trial data revealed strong correlation between ePRA and [Delta]rpcPRA (r=0.817)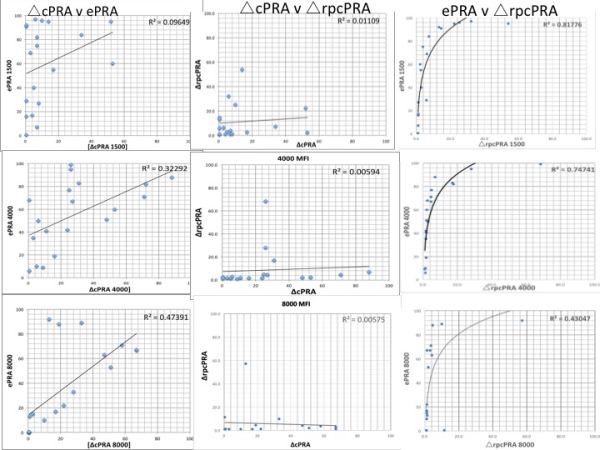 .
.
CONCLUSIONS
Analyses indicate that ePRA and rpcPRA provide superior sensitivity to cPRA in DS, and are promising potential endpoints in DS trials.
CITATION INFORMATION: Woodle E, Pando Rigal M, Tremblay S, Girnita A. Enabled PRA and Precision PRA: Alternatives to cPRA as Desensitization Trial Endpoints. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Woodle E, Rigal MPando, Tremblay S, Girnita A. Enabled PRA and Precision PRA: Alternatives to cPRA as Desensitization Trial Endpoints. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/enabled-pra-and-precision-pra-alternatives-to-cpra-as-desensitization-trial-endpoints/. Accessed July 18, 2025.« Back to 2016 American Transplant Congress
