Distinct Delayed Classical Complement Pathway Activation Highlights Transition of Ischemic Acute Kidney Injury to Chronic Kidney Disease with Interstitial Fibrosis/Tubular Atrophy
Comprehensive Transplant Center, Cedars Sinai Medical Ctr, Los Angeles, CA
Meeting: 2019 American Transplant Congress
Abstract number: A110
Keywords: Graft function, Ischemia, Kidney, Kidney transplantation
Session Information
Session Name: Poster Session A: Ischemia Reperfusion & Organ Rehabilition
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: Ischemia reperfusion Injury (IRI)-induced AKI of the renal allograft frequently leads to delayed graft function (DGF). Patients with DGF suffer from prolonged hospitalization with increased morbidity and experience long-term increased risk of graft loss compared to no DGF. Currently, no definite therapies exist to treat DGF and its sequelae per se. Complement activation significantly contributes to the initiation of murine DGF. Recently, in a double-blind, placebo-controlled trial of C1 esterase inhibitor to prevent DGF, we reported significant reductions in need for dialysis during the first week after deceased donor kidney transplantation (KTx) in patients who received C1NH intra-operative and 24 hours after KTx. Whether complement inhibition may retard the progression of established DGF is not known.
*Methods: Mining of recently published RNA-Seq-based datasets of our thoroughly characterized, robust, long-term survival compatible (up to 1 year) renal IRI induced AKI to CKD (IF/TA) mice model.
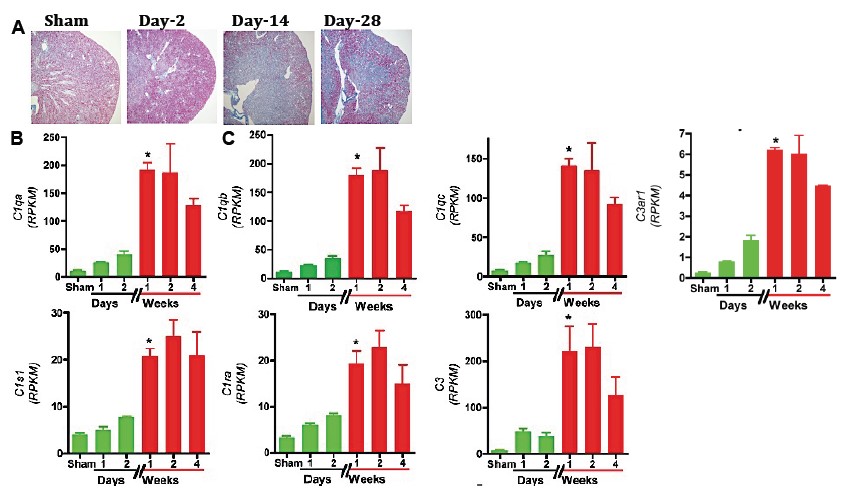
*Results: RNA-Seq based transcriptomic analysis of the kidneys showed an expected early complement pathway activation after injury: C1qa [3.6-fold (x)], C1qb (2.8x), C1qc (3.6x), C3ar1 (2.3x), C1s1 (1.9x), C1ra (2.4x), and C3 (5.2x) transcript induction by 48h after injury compared to sham surgery controls. However, unexpectedly we identify a further striking induction of all the above complement components at day-7 after injury, which persisted till day-14 coinciding with the transition of AKI to iIF/TA: C1qa (17.4x vs. sham; 4.7x vs. 48h), C1qb (14.8x vs. sham; 5x vs. 48h), C1qc (18.5x vs. sham; 5x vs. 48h), C1s1 (5.2x vs. sham; 2.7x vs. 48h), C1ra (5.7x vs. sham; 2.3x vs. 48h), C3ar1 (7.7x vs. sham; 3.3x vs. 48h), and C3 (30x vs. sham; 5.7x vs. 48h) transcripts (See below Figure).
*Conclusions: Our findings unravel previously unknown a distinct, delayed, C1-driven, classical complement pathway as a high-value target with a rapid bench to bedside translational promise for retarding the progression of iIF/TA after ischemic AKI. The clinical relevance and cellular source of this “delayed complement storm” is being elucidated in our animal and human studies.
To cite this abstract in AMA style:
Kumar S, Jordan S. Distinct Delayed Classical Complement Pathway Activation Highlights Transition of Ischemic Acute Kidney Injury to Chronic Kidney Disease with Interstitial Fibrosis/Tubular Atrophy [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/distinct-delayed-classical-complement-pathway-activation-highlights-transition-of-ischemic-acute-kidney-injury-to-chronic-kidney-disease-with-interstitial-fibrosis-tubular-atrophy/. Accessed February 22, 2026.« Back to 2019 American Transplant Congress