De Novo NAFLD Post-Liver Transplant is Defined by Altered Lipid Metabolism and Mitochondrial Dysfunction
1Multi-Organ Transplant, UHN, Toronto, ON, Canada, 2Division of Gastroenterology and Hepatology, UHN, Toronto, ON, Canada
Meeting: 2020 American Transplant Congress
Abstract number: 156
Keywords: Immunosuppression, Lipids, Liver transplantation, Post-operative complications
Session Information
Session Name: Liver: Immunosuppression and Rejection
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:15pm-4:27pm
Presentation Time: 4:15pm-4:27pm
Location: Virtual
*Purpose: De Novo Non-alcoholic Fatty Liver Disease (NAFLD) affects up to 35% of liver transplant (LT) recipients transplanted for non-NAFLD indications. Non-alcoholic steatohepatitis (NASH), the more severe form of NAFLD, is associated with increased cardiovascular morbidity and graft failure. The aim of our study was to perform molecular characterization of De Novo NASH for the first time and to compare and contrast with non-transplant NASH.
*Methods: We collected publicly available high throughput gene expression data in NASH patients from Gene Expression Omnibus (GSE63067). Gene expression profiling was performed on liver biopsies from five De Novo NAFLD transplant patients using GeneChipTM Human Genome U133 Plus 2.0 Array platform compared to five healthy controls. Pathway enrichment analysis and upstream regulators in the De Novo NAFLD samples were identified by Ingenuity pathway analysis (IPA) software. Pathway analysis for the common and the unique dysregulated genes in NAFLD and De Novo NAFLD was obtained by pathDIP. An integrative analysis of the dysregulated genes and the enriched pathways was performed and visualized as a network using NAViGaTOR.
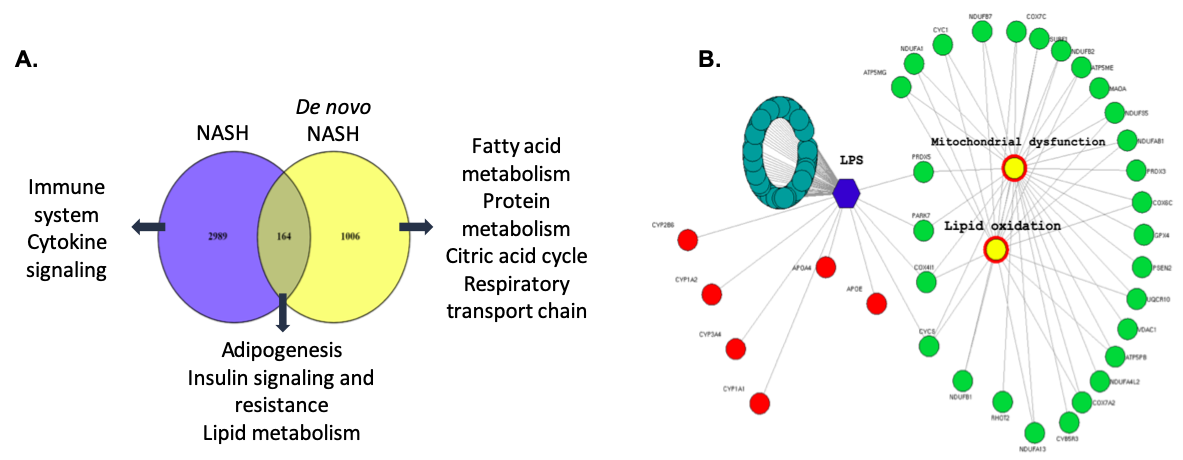
*Results: The venn diagram identified 1006 genes specific to De Novo NASH patients; they were highly associated with protein and fatty acid metabolism, the citric acid cycle and respiratory electron transport. Mitochondrial dysfunction was the most significant pathway; oxidative phosphorylation was significantly activated. 2989 dysregulated genes specific to non-transplant NASH patients were highly involved in immune system and cytokine signaling. 164 overlapping genes between non-transplant NASH and De Novo NASH were associated with adipogenesis, insulin resistance and lipid metabolism were upregulated in common (Figure 1). Lipopolysaccharide (LPS) was identified by IPA as a central upstream regulator activated in De Novo NASH leading to a dysregulation of several genes involved in lipid metabolism as well as lipid oxidation and mitochondrial dysfunction.
*Conclusions: Mitochondrial function, respiration and oxidative phosphorylation were processes specific to De Novo NASH. Pathways involved in hepatic lipogenesis and insulin resistance were upregulated in De Novo NASH post-LT. LPS is an upstream regulator of these pathways, and may play a role in upregulation of metabolic genes in De Novo NASH. Our study represents the first examination of understanding the molecular basis of De Novo NAFLD, to prevent long-term complications post-LT.
To cite this abstract in AMA style:
Bhat M, Pasini E, Angeli M, Allard J, Humar A. De Novo NAFLD Post-Liver Transplant is Defined by Altered Lipid Metabolism and Mitochondrial Dysfunction [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/de-novo-nafld-post-liver-transplant-is-defined-by-altered-lipid-metabolism-and-mitochondrial-dysfunction/. Accessed July 3, 2025.« Back to 2020 American Transplant Congress