Conversion from Tacrolimus (TAC) to Sirolimus (SLR)-Based Immunosuppression in Pediatric Patients Who Underwent Liver Transplantation for Unresectable Hepatoblastoma – 2 Year Follow-Up.
1Pediatric/Transplant Surgery, Children's Mercy Hospitals and Clinics, Kansas City, MO
2Hepatology, Children's Mercy Hospitals and Clinics, Kansas City, MO.
Meeting: 2016 American Transplant Congress
Abstract number: D214
Keywords: Immunosuppression, Liver transplantation, Pediatric, Sirolimus (SLR)
Session Information
Session Name: Poster Session D: Pediatric Liver Transplantation
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Introduction: The appropriate use of Sirolimus (SRL) immunosuppression in pediatric liver transplantation has yet to be clearly defined. Even though Tacrolimus (TAC) is commonly used for immunosuppression, a side effect is nephrotoxicity. We reported that early conversion from TAC to SRL was safe, effective and beneficial in this subset of liver transplant recipients who have undergone Cisplatin based chemotherapy.
Methods: Between February 2008 – May 2013, 6 patients were transplanted for unresectable hepatoblastoma (HB). All patients received at least one course of Cisplatin-based chemotherapy prior to transplant. Indications for conversion were: 1.) protect renal function given the known renal toxicity of Cisplatin, and 2.) potential anti-proliferative (anti-tumor) effects of SRL. Monitoring of patient labs, mGFR and tumor recurrence were conducted routinely through November 2015.
Results: All patients were switched from TAC to SRL within the first year after transplant. One patient moved out of state and transferred to another program. The 5 remaining patients had improvement of their mGFRs from baseline (see figure). To date, no discontinuation was required due to side effects or persistent rejection episodes. We identified two patients with elevated AFP post transplant, one with radiographically confirmed metastatic lung recurrence and the other with no identifiable source.
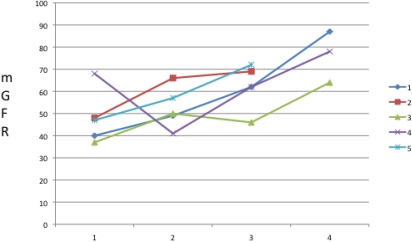
Conclusions: Converting immunosuppression from TAC to SRL is safe and effective in this selected high risk group of pediatric liver transplant recipients. This two year follow-up demonstrated 100% improvement in mGFR. We noted an elevated AFP in two patients, with radiographic and biopsy proven metastatic lung recurrence in one and no obvious source in the other. While continued monitoring in this high risk group of pediatric transplant patients is imperative, SRL-based immunosuppression may improve renal outcomes and limit tumor recurrence.
CITATION INFORMATION: Hendrickson R, Andrews W, Davis M, Cisneros R, Fischer R, Daniel J. Conversion from Tacrolimus (TAC) to Sirolimus (SLR)-Based Immunosuppression in Pediatric Patients Who Underwent Liver Transplantation for Unresectable Hepatoblastoma – 2 Year Follow-Up. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Hendrickson R, Andrews W, Davis M, Cisneros R, Fischer R, Daniel J. Conversion from Tacrolimus (TAC) to Sirolimus (SLR)-Based Immunosuppression in Pediatric Patients Who Underwent Liver Transplantation for Unresectable Hepatoblastoma – 2 Year Follow-Up. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/conversion-from-tacrolimus-tac-to-sirolimus-slr-based-immunosuppression-in-pediatric-patients-who-underwent-liver-transplantation-for-unresectable-hepatoblastoma-2-year-follow-up/. Accessed July 3, 2025.« Back to 2016 American Transplant Congress
