Comparison of Prophylaxis Strategies for CMV Infection in Liver Transplant Recipients
K. Schnelle, A. Leino, M. Chunduru, L. Von Stein, O. Witkowsky
The Ohio State Wexner Medical Center, Columbus, OH
Meeting: 2020 American Transplant Congress
Abstract number: B-183
Keywords: Cytomeglovirus, Infection, Liver transplantation, Viral therapy
Session Information
Session Name: Poster Session B: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: The best strategy to prevent cytomegalovirus (CMV) following liver transplantation is not defined for intermediate risk recipients (recipient seropositive (R+) regardless of donor status). The purpose of this study was to compare outcomes associated with no prophylaxis to prophylactic valganciclovir (VGCV) in R+ recipients.
*Methods: This retrospective evaluation included adult liver transplant recipients with R+ CMV serostatus transplanted from August 1, 2016 to August 6, 2018. Patients were excluded if initiated on everolimus in the first 90-days post-transplantation. Prior to 1/1/2018 CMV prophylaxis was not used; after 1/1/2018 VGCV prophylaxis was initiated for 90 days. The primary outcome was incidence of CMV infection defined as CMV above the lower limit of detection on plasma PCR. Secondary outcomes included assessment of CMV disease, the incidence of leukopenia, and frequency of mycophenolate dose modification for leukopenia.
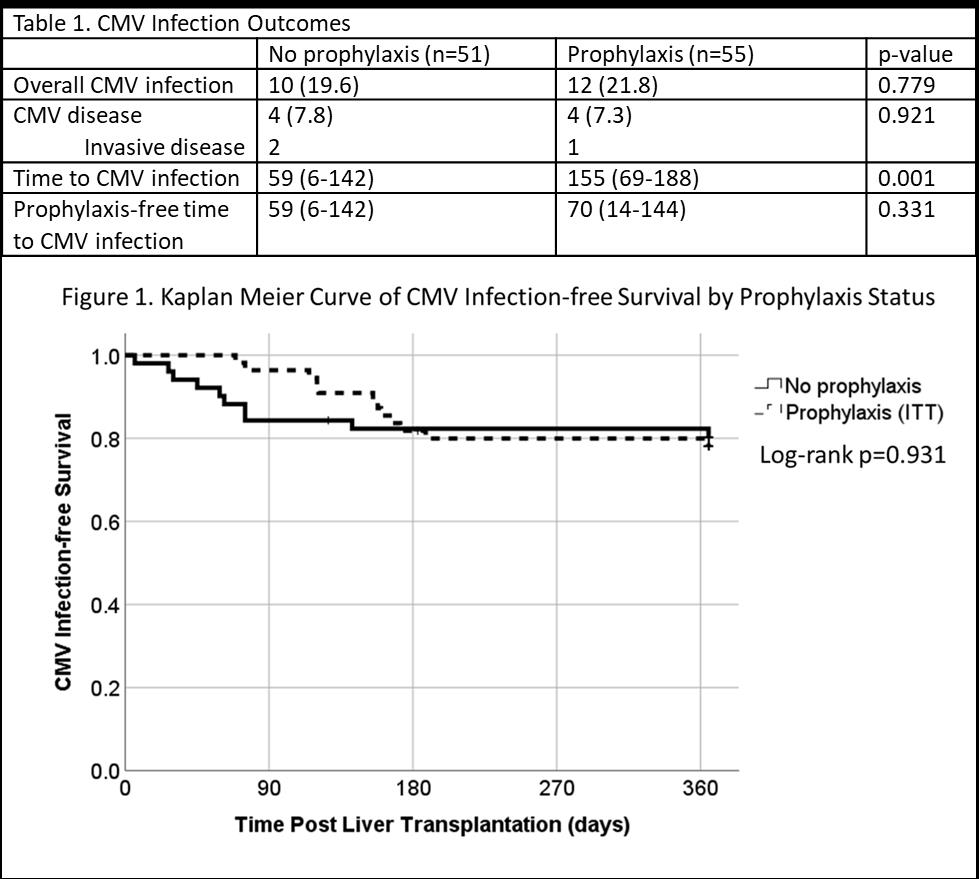
*Results: Overall incidence of CMV infection in no prophylaxis group was 19.6% compared to 26.4% in the VGCV prophylaxis group (p=0.779). VGCV delayed the time to CMV infection onset in univariate analysis (Table 1) but there was no difference by log-rank test with patients developing CMV at a similar rate following cessation of prophylaxis (Figure 1). No patients experienced breakthrough CMV infection (patients in the VGCV group with CMV infection prior to day 90 had VGCV held for leukopenia). The rate of CMV disease was low in both groups (Table 1). CTCAE grade ≥3 leukopenia (WBC <2k/µL) in the first 90 days post-transplant occurred more frequently in the VGCV cohort (27.5% vs 52.7%, p=0.008). Additionally, VGCV increased the rate of mycophenolate modification (reduced or held) for leukopenia in the first 90 days (33.3% vs 52.7%, p=0.044). To understand the potential impact of leukopenia and/or mycophenolate reduction an unpowered, post-hoc analysis of the rate of rejection and infection was conducted. The rejection occurred in 14.3% with no prophylaxis vs 18.9% with VGCV prophylaxis (p=0.523). There was no difference in the incidence of non-CMV infection (29.4% no prophylaxis vs 30.9% VGCV prophylaxis, p=0.867).
*Conclusions: In the studied cohort, there was no difference in the incidence of CMV infection with the addition of VGCV prophylaxis in R+ liver recipients. The implications of the increased rate of severe leukopenia with VGCV prophylaxis should be further investigated.
To cite this abstract in AMA style:
Schnelle K, Leino A, Chunduru M, Stein LVon, Witkowsky O. Comparison of Prophylaxis Strategies for CMV Infection in Liver Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/comparison-of-prophylaxis-strategies-for-cmv-infection-in-liver-transplant-recipients/. Accessed March 2, 2026.« Back to 2020 American Transplant Congress