CMV IgG Provides Similar Anti-CMV Efficacy as Valganciclovir in High-Risk Organ Transplant Recipients, With Reduced Costs
N. Pilch, D. Taber, C. Schaffner, S. Nadig, J. McGillicuddy, C. Bratton, P. Baliga, K. Chavin.
MUSC, Charleston, SC.
Meeting: 2015 American Transplant Congress
Abstract number: D248
Keywords: Cytomeglovirus, Immunoglobulins (Ig)
Session Information
Session Name: Poster Session D: Viral Infections
Session Type: Poster Session
Date: Tuesday, May 5, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Abdominal organ transplants at high risk for CMV infection (D+/R-) remain a difficult cohort to prevent late CMV infection despite prolonged valganciclovir (VGC) administration. Administration of passive immunity using CMV hyperimmune globulin (CMV-IgG) may provide adequate prophylaxis and allow for development of active immunity in these at risk patients. The aim of this study was to evaluate VCG for 100 days post-transplant in combination with CMV-IgG versus VCG for 200 days in D+/R- abdominal transplant recipients.
Methods: Prospective, randomized, open label pilot study of 40 consecutively enrolled adult abdominal transplant recipients. Patients were randomized and stratified based on organ to VCG per package insert guidelines for 200 days vs VCG for 100 days post-transplant with CMV-IgG 100 mg/kg administered at 90 days, 120 days, and 180 days post-transplant. The primary outcome was incidence and severity of late CMV disease, defined as CMV syndrome or tissue invasive disease occurring between 100 and 200 days and after 200 days post-transplant.
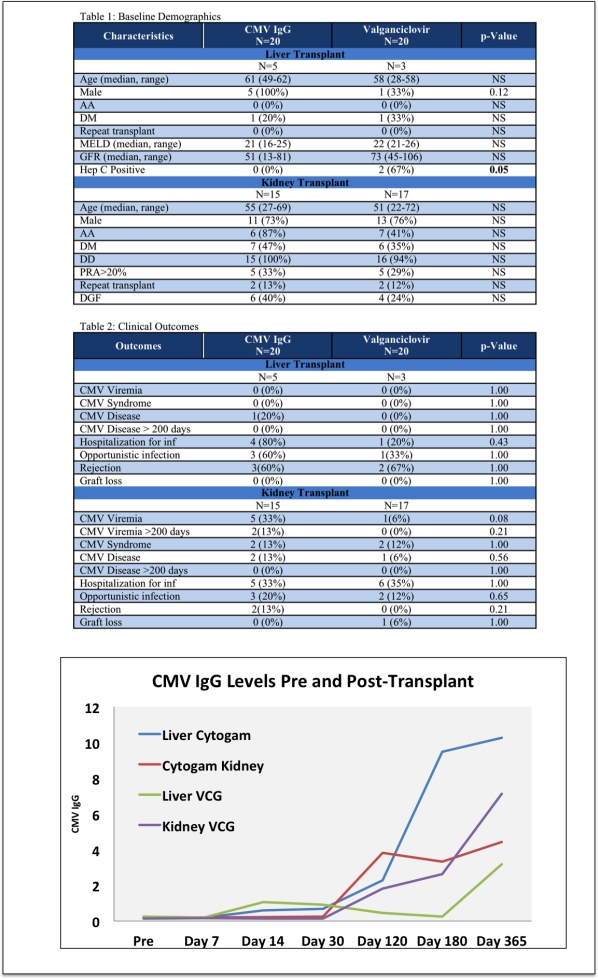
Results: Of the 40 patients enrolled, a total of 6 patients developed CMV viremia. Viremia occurred in 2 patients in the CMV IgG group after 200 days (216 and 260 days post-transplant). There were similar rates of opportunistic infections and hospitializations in the kidney recipients between groups, but numerically more in the liver CMV IgG group (see Table 2). CMV syndrome and disease before and after 200 days were modest in all groups (Table 2).
Conclusion: CMV IgG provides a reasonable alternative to VCG in high risk abdominal organ transplant recipients and may be beneficial when VCG is cost prohibitive or patients develop intolerance due to cytopenias. Larger trials are needed to confirm these results.
To cite this abstract in AMA style:
Pilch N, Taber D, Schaffner C, Nadig S, McGillicuddy J, Bratton C, Baliga P, Chavin K. CMV IgG Provides Similar Anti-CMV Efficacy as Valganciclovir in High-Risk Organ Transplant Recipients, With Reduced Costs [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/cmv-igg-provides-similar-anti-cmv-efficacy-as-valganciclovir-in-high-risk-organ-transplant-recipients-with-reduced-costs/. Accessed July 12, 2025.« Back to 2015 American Transplant Congress
