Cardiac Grafts from Insulin Resistant Donors Trigger a Heightened Immune Response via Excessive Induction of Autophagy
1Transplantation Research Center, Renal Division, Brigham and Women's Hospital, Boston, MA
2Division of Endocrinology, Boston Children's Hospital, Boston, MA.
Meeting: 2018 American Transplant Congress
Abstract number: A72
Keywords: Graft arterlosclerosis, Heart/lung transplantation, Ischemia, Obesity
Session Information
Session Name: Poster Session A: Innate Immunity; Chemokines, Cytokines, Complement
Session Type: Poster Session
Date: Saturday, June 2, 2018
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall 4EF
Background Heart transplantation remains the treatment of choice for many patients with medically refractory heart failure. The use of organs from patients with insulin resistant diabetes has been found to be an independent risk factor for mortality.
Methods Donor c57BL/6 (B6) mice were fed a high fat diet to induce obesity. Cardiac grafts were preserved for 6 hours at 0[deg]C (CI 6hr) or transplanted immediately (no CI) into lean B6 mice. Recipients' spleens were harvested one-day post transplantation (POD1) for flow cytometry (FCM). Grafts were isolated on POD1 for gene profiling.
Results mRNA expression by qRT-PCR showed upregulation of the hypertension associated CVD related gene: Atgr1a Cxcr2 as well as the hyperlipidemia associated CVD related gene: Fmo3 furthermore Foxo1 was also significantly higher in obese mice heart at the of harvesting. The proinflammatory cytokines: IL-6st, IL-1β, TNF-α and IFN-γ were also increased in obese mice hearts. Moreover, the gene expression of transcriptional factors T-bet, ROR-α and ROR-γt, markers of T cells activation were increased in obese mice hearts. More surprisingly, the mRNA expression of autophagy related genes Atg2a, Atg3, Atg4a, Atg7, Atg10, Atg12 and PDK4 increased in obese mice hearts.
FCM of recipients' splenocytes showed that the frequency of effector CD4+ T cells, CD8+ cytotoxic T cells increased in obese donor group. In addition, both CD4+ and CD8+ T cells derived IFN-γ and IL17A increased in the obese group, indicating that not only the number, but also the activity of recipient T cells is enhanced after implantation of the obese grafts.
Lastly, graft mRNA expression indicated that the Treg related gene Foxp3 was lower and the Teff related gene T-bet, ROR-α and ROR-γt was higher in the obese donor group. The trend of CVD related-, proinflammatory cytokines and autophagy related gene expression was higher in obese grafts.
Conclusions Cardiac grafts from insulin resistant donors activate endocrine-immune crosstalk to trigger a heightened immune response via excessive induction of autophagy.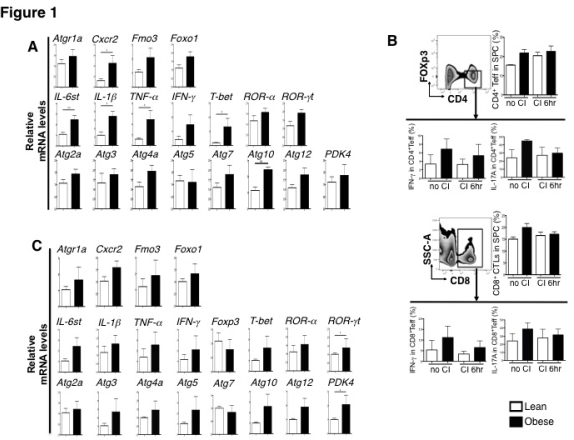
CITATION INFORMATION: Cai S., Miao J., Chandraker A. Cardiac Grafts from Insulin Resistant Donors Trigger a Heightened Immune Response via Excessive Induction of Autophagy Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Cai S, Miao J, Chandraker A. Cardiac Grafts from Insulin Resistant Donors Trigger a Heightened Immune Response via Excessive Induction of Autophagy [abstract]. https://atcmeetingabstracts.com/abstract/cardiac-grafts-from-insulin-resistant-donors-trigger-a-heightened-immune-response-via-excessive-induction-of-autophagy/. Accessed March 7, 2026.« Back to 2018 American Transplant Congress
