Blockade of HLA Antibody-Triggered Classical Complement Activation by High-Affinity Humanized Monoclonal Anti-C1s Antibody TNT009 – Results of a First-in-Human Phase 1 Trial.
1Department of Surgery, Medical University of Vienna, Vienna, Austria
2Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Austria
3Division of Nephrology and Dialysis, Department of Medicine III, Medical University of Vienna, Vienna, Austria
4True North Therapeutics, Inc., South San Francisco, CA.
Meeting: 2016 American Transplant Congress
Abstract number: 414
Keywords: Dosage, HLA antibodies, Immunosuppression, Rejection
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Novel Agents
Session Type: Concurrent Session
Date: Tuesday, June 14, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:54pm-3:06pm
Presentation Time: 2:54pm-3:06pm
Location: Veterans Auditorium
Study purpose: Classical complement may play a key role in ABMR. A promising therapeutic approach could be complement inhibition at the level of key component C1. In this single ascending dose part of a first-in-human phase 1 trial (NCT02502903) we evaluated the tolerability and activity of TNT009, a high-affinity humanized monoclonal antibody directed against serine protease C1s. Methods: In a double-blind, randomized, placebo-controlled phase 1 trial, 48 healthy volunteers received either a single dose of TNT009 or placebo by IV infusion (3:1 randomization; 7 cohorts: 0.3-100 mg/kg). To determine the effect of TNT009 on ex vivo HLA antibody-triggered complement activation, serum samples from dosed subjects were added as a complement source in a modified solid phase assay. C3d deposition was detected on HLA haplotype-coated flow beads pre-incubated with pooled inactivated sera obtained from sensitized patients. Results were expressed as the mean of C3d MFI determined on 16 defined bead populations. Results. Single doses of 3-100 mg/kg TNT009 led to a >85% inhibition of HLA antibody-triggered C3d bead deposition. At doses of 10, 30, 60 or 100 mg/kg this effect lasted for 2 to at least 14 days. 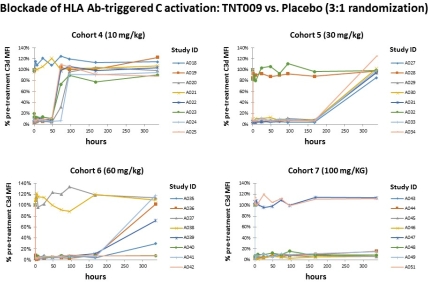 C3d test results showed a tight dose-effect relationship without major inter-individual differences. Similarly, in vitro spiking of sera with TNT009 revealed uniform and strong complement inhibition at concentrations >30 [mu]g/mL. Conclusion. We demonstrate that TNT009 allows for prolonged and complete classical pathway inhibition in vivo. Future studies will clarify whether TNT009 is able to block antibody-mediated injury as a strategy to prevent or treat ABMR.
C3d test results showed a tight dose-effect relationship without major inter-individual differences. Similarly, in vitro spiking of sera with TNT009 revealed uniform and strong complement inhibition at concentrations >30 [mu]g/mL. Conclusion. We demonstrate that TNT009 allows for prolonged and complete classical pathway inhibition in vivo. Future studies will clarify whether TNT009 is able to block antibody-mediated injury as a strategy to prevent or treat ABMR.
CITATION INFORMATION: Mühbacher J, Jilma B, Wahrmann M, Marinova L, Eskandary F, Gilbert J, Panicker S, Böhmig G. Blockade of HLA Antibody-Triggered Classical Complement Activation by High-Affinity Humanized Monoclonal Anti-C1s Antibody TNT009 – Results of a First-in-Human Phase 1 Trial. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Mühbacher J, Jilma B, Wahrmann M, Marinova L, Eskandary F, Gilbert J, Panicker S, Böhmig G. Blockade of HLA Antibody-Triggered Classical Complement Activation by High-Affinity Humanized Monoclonal Anti-C1s Antibody TNT009 – Results of a First-in-Human Phase 1 Trial. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/blockade-of-hla-antibody-triggered-classical-complement-activation-by-high-affinity-humanized-monoclonal-anti-c1s-antibody-tnt009-results-of-a-first-in-human-phase-1-trial/. Accessed July 18, 2025.« Back to 2016 American Transplant Congress
