Anti-Thymocyte Globulin Promotes Tfh Cells Differentiation and Alloantibody Production
Transplantation Research Center, Brigham and Women's Hospital, Boston, MA
Meeting: 2020 American Transplant Congress
Abstract number: 542
Keywords: Antibodies, B cells, Heart/lung transplantation, T helper cells
Session Information
Session Name: B-cells / Antibodies /Autoimmunity
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:39pm-3:51pm
Presentation Time: 3:39pm-3:51pm
Location: Virtual
*Purpose: Antibody-mediated rejection is a major cause of long-term graft loss. T follicular helper (Tfh) cell is CD4+ T cell subset that is specialized in providing help for germinal center (GC) reactions where B cells are activated, differentiate and produce high-affinity antibodies. Anti-thymocyte globulin (ATG) is a widely used lymphocyte-depleting induction therapy, however, little is known about how ATG affects Tfh development and DSA formation.
*Methods: To study the effect of ATG on Tfh, we developed mouse ATG (mATG) by immunizing rabbit with mouse thymocytes and tested mATG in a fully MHC allogeneic transplant model and in a NP-Ova/CFA immunization model. In the latter, we immunized mice with NP-Ova (100 mcg/mouse, s.c.) and CFA on day 0, and received IgG control or mATG (500 mcg/mouse, i.p.) on day 0 and day 4. We also analyzed PBMCs for Tfh frequency in transplant recipients that had received ATG vs. no-ATG as induction.
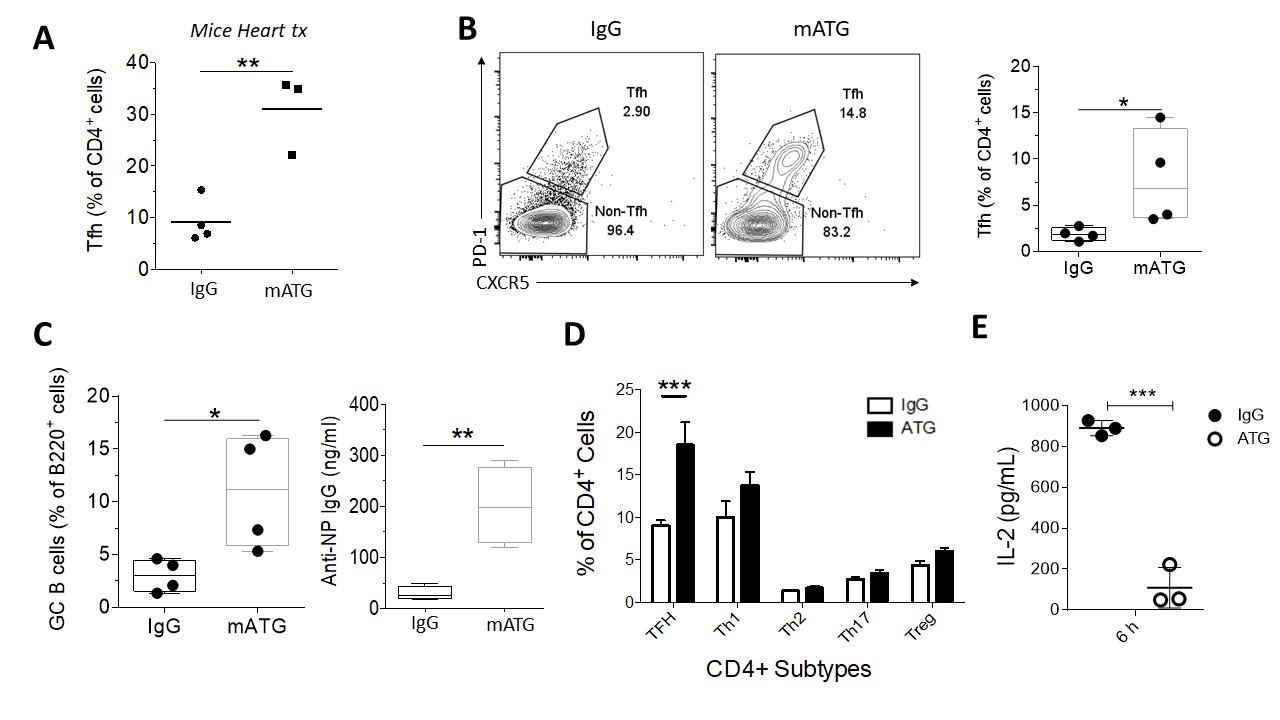
*Results: We observed higher circulating Tfh (cTfh) in transplant patients treated with ATG. In mice, mATG effectively depleted the peripheral CD4+ and CD8+ T cells in spleen, lymph nodes (LNs) and peripheral blood in naïve mice. Depletion was less effective in Tfh and Treg cells. In full HLA-mismatch cardiac transplant model, mATG treated group had higher percentage of Tfh in peripheral blood, consistent with human data. To dissect the exact mechanism of higher percentage of remaining Tfh, we used NP-Ova/CFA immunization model. mATG-treated group had higher percentage of Tfh cells (CD4+CXCR5+PD-1+) (Fig.1 B), GC B cells (B220+Fas+GL7+) (Fig.1 C), and higher titer of NP-specific antibody (Fig.1 C) compared to controls. We also evaluated other CD4+ T cells subpopulations by staining for transcriptor factors T-Bet (Th1 cells), GATA3 (Th2 cells), RORyT (Th17 cells), Foxp3 (T regulatory cells) and the dominant difference were in Bcl-6 (Tfh cells) in all tissues analyzed (Fig.1 D). Lastly, the Bcl-6 repressor cytokine IL-2 was decreased 6h after the ATG administration in serum (Fig.1 E). This suggests that less IL-2 availability caused by mATG creates favorable environment for Tfh differentiation
*Conclusions: mATG is effective in depleting T cells. However, the remaining CD4+ T cells are mostly Tfh cells. Lower IL-2 appears to favor Tfh cells differentiation, although the mechanisms require further investigation. Since antibody-mediated rejection is a dominant cause of graft failure, investigating the mechanism of this resistance is crucial for the development of more effective therapies in restraining Tfh cells.
To cite this abstract in AMA style:
Gassen RBenedetti, Aoyama B, Lima M, Borges T, Pérez-Sáez M, Murakami N, Sage P, Pascual J, Riella LV. Anti-Thymocyte Globulin Promotes Tfh Cells Differentiation and Alloantibody Production [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/anti-thymocyte-globulin-promotes-tfh-cells-differentiation-and-alloantibody-production/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress