AMD3100 (Plerixafor) as a Single-Dose Stem Cell Mobilizing Agent in Vascularized Composite Tissue Allograft (VCA) Transplantation in a Canine DLA-Mismatch Model
1University of Colorado School of Medicine, Aurora, CO, 2Fred Hutchinson Cancer Research Center, Seattle, WA
Meeting: 2019 American Transplant Congress
Abstract number: A70
Keywords: Engraftment, Graft acceptance, Stem cells, Tolerance
Session Information
Session Name: Poster Session A: Basic & Clinical Science – VCA
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: Vascularized Composite Allograft (VCA) transplantation is a clinical reality but limited by toxicities of chronic immunosuppression and rejection. Current clinical tolerance protocols rely on recipient conditioning and donor cell mobilization that limits use to living donor transplants. We sought to design a clinically relevant protocol applicable to cadaveric organs. We previously demonstrated that using AMD3100 (Plerixafor) as a single dose agent for stem cell mobilization was successful in a DLA-haploidentical model. We wanted to increase clinical relevance by testing our existing non-myeloablative stem cell canine VCA transplant model to DLA-mismatched, unrelated canine donor-recipient pairs.
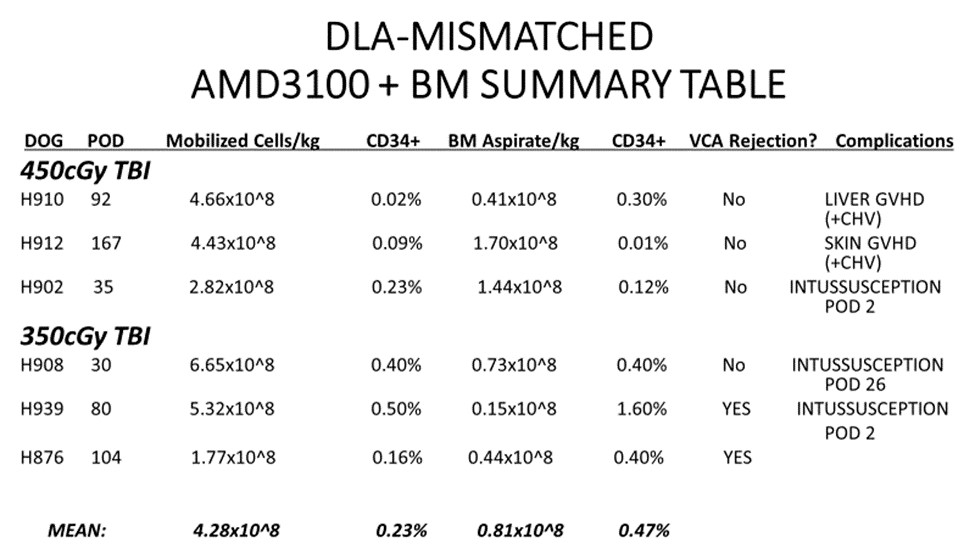
*Methods: Three DLA-mismatched, unrelated canine recipients [Group I] received conditioning with 450cGy TBI, AMD3100-mobilized donor stem cells + Bone Marrow (BM) infusion and simultaneous VCA transplantation with a short course of immunosuppression (Sirolimus: 28 days/MMF: 56 days/CSP: 70 days). Three DLA-mismatched, unrelated canine recipient [Group II] underwent a less intense conditioning regimen (350cGy TBI) but otherwise identical transplantation protocol. CD34+ hematopoietic progenitor cells were quantified via flow cytometry. Peripheral blood chimerism was evaluated by PCR techniques weekly. VCA graft survival was followed clinically and histologically.
*Results: All six canines tolerated the conditioning regimen. Stem cell engraftment and donor chimerism was seen in all dogs. Mean COBE apheresis count was 4.28×10^8 cells/kg and mean BM aspirate count was 0.81×10^8 cells/kg across both groups. Outcomes varied. No evidence of acute rejection was seen. Two dogs demonstrated signs of VCA rejection once off immunosuppression. GVHD (skin and/or liver) was seen in two dogs. Two dogs were lost post-operatively to the unexpected complication of intussusception while still seemingly tolerant to the VCA.
*Conclusions: This study demonstrates proof of principle for AMD3100 as a single-dose stem cell mobilizing agent for a clinically relevant tolerance protocol in mismatched, unrelated donor-recipient pairs. Use of AMD3100 led to stem cell engraftment in all animals transplanted with no evidence of acute rejection in the VCA. AMD3100 use limited by thrombocytopenia in our previous studies continue to appear be resolved with the addition of BM Aspirate in this model. Continued experiments should allow for longer-term follow up in future canine recipients that should optimistically not experience bowel complications or GVHD.
To cite this abstract in AMA style:
Swearingen B, Graves S, Storb R, Mathes DW. AMD3100 (Plerixafor) as a Single-Dose Stem Cell Mobilizing Agent in Vascularized Composite Tissue Allograft (VCA) Transplantation in a Canine DLA-Mismatch Model [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/amd3100-plerixafor-as-a-single-dose-stem-cell-mobilizing-agent-in-vascularized-composite-tissue-allograft-vca-transplantation-in-a-canine-dla-mismatch-model/. Accessed February 22, 2026.« Back to 2019 American Transplant Congress