AMD3100 and Low-Dose FK506 Prevent De Novo Donor Specific Antibody Formation and Rejection in a Sensitized Kidney Transplantation Model
Johns Hopkins University School of Medicine, Baltimore, MD
Meeting: 2019 American Transplant Congress
Abstract number: A50
Keywords: Alloantibodies, Graft survival, Kidney/liver transplantation, Sensitization
Session Information
Session Name: Poster Session A: B-cell / Antibody /Autoimmunity
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: De novo donor specific antibody (DSA) formation and antibody mediated rejection (AMR) are the main cause of allograft failure long-term. We have developed a novel stem cell mobilizing strategy that enables long-term liver and kidney allograft survival without sustained immunosuppression in small and large animals using a safe combination of two FDA approved drugs AMD3100 and low-dose FK506 (AF). Furthermore no increase in serum DSA levels was detected in animals displaying long-term kidney allograft survival even after donor skin grafts were rejected. Therefore the purpose of this study is to determine if AF combination treatment prevents de novo DSA production in a rat model of skin allograft sensitization and kidney transplantation.
*Methods: Skin allografts from Dark Agouti rats were transplanted onto adult Lewis recipients. Recipient animals were randomly divided into AF treatment group (AMD3100 1mg/kg and FK506 0.1mg/kg, sc, every other day for 14 days) and control group (same volume of saline, sc). Serum levels of DSAs IgG and IgM were measured by flow cytometry. The sensitization to donor antigens in Lewis rats at 2 months after skin allografting was further confirmed by transplanting donor DA kidneys. FK506 (1mg/kg/day, sc) was given to prevent acute cellular rejection after kidney transplantation.
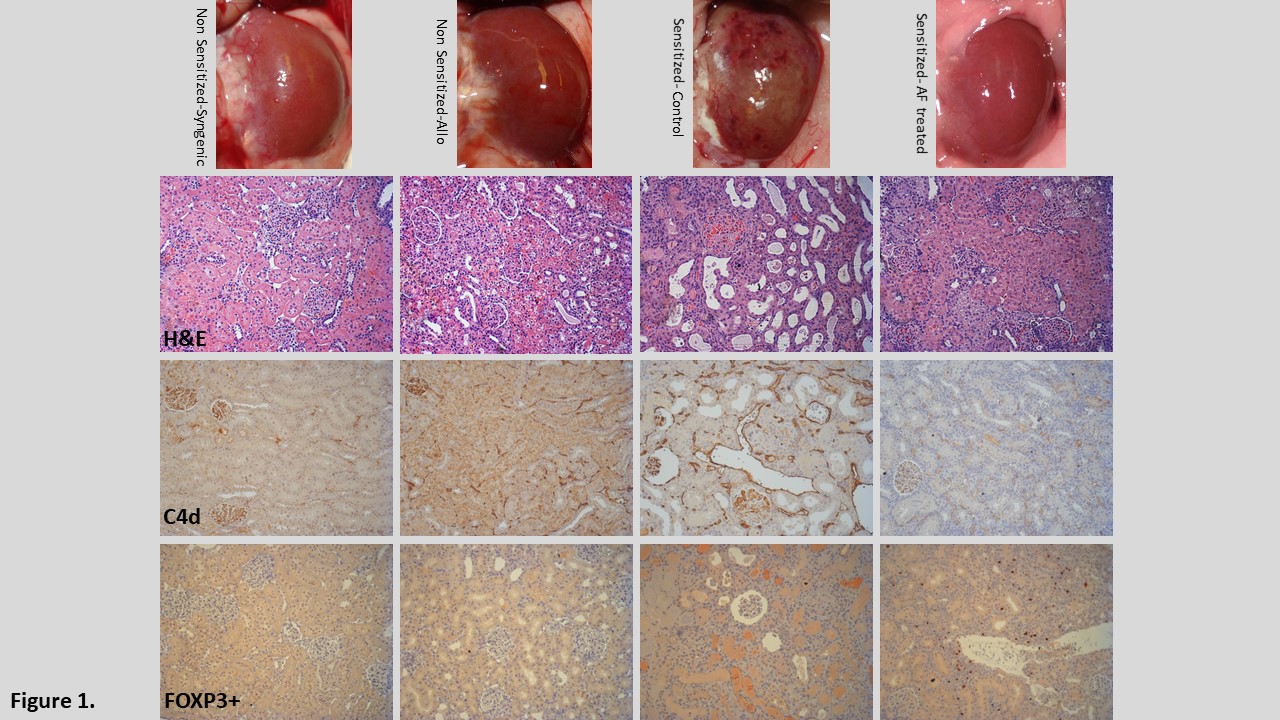
*Results: Skin allografts were rejected in 8-10 days after transplantation in both groups. AF treated animals showed significantly higher presence of FOXP3+ in rejected skin allografts. Serum levels of DSA-IgG were significantly lower at 2 and 4 weeks between the control (N=13) and treated animals (N=11) (p<0.05) but recovered to comparable levels at 8 weeks after skin transplantation. All control animals (n=5) died within 7 days after kidney transplantation. DSA deposition and AMR in kidney allografts were confirmed by histological studies (Fig1). In contrast, about 64% (7/11) animals with AF treatment after skin allografting survived over three weeks without AMR and no increase in serum levels of DSA at 14 days after kidney transplantation. Sensitized animals treated with AF showed more FOXP3+ cells in transplanted kidneys (Fig1) and a lower number of CD138+ long lived plasma cells in the spleen.
*Conclusions: AF combination therapy significantly reduced de novo DSA production after skin rejection and prevented acute AMR of the kidney allograft by mobilizing antigen specific FOXP3+ regulatory T-cells to the graft. This treatment may be a viable alternative strategy to conventional immunosuppressive regimens.
To cite this abstract in AMA style:
Ahmadi AR, Qi L, Wesson RN, Cameron AM, Sun Z. AMD3100 and Low-Dose FK506 Prevent De Novo Donor Specific Antibody Formation and Rejection in a Sensitized Kidney Transplantation Model [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/amd3100-and-low-dose-fk506-prevent-de-novo-donor-specific-antibody-formation-and-rejection-in-a-sensitized-kidney-transplantation-model/. Accessed March 3, 2026.« Back to 2019 American Transplant Congress