Alloimmune Recognition Blocks Recipient Macrophage Differentiation into Reparative Subsets
1Immunology, Thomas E. Starzl Transplantation Institute, University at Pittsburgh, Pittsburgh, PA, 2Surgery, Thomas E. Starzl Transplantation Institute, Pittsburgh, PA
Meeting: 2022 American Transplant Congress
Abstract number: 1241
Keywords: Allorecognition, Effector mechanisms, Immune deviation, Inflammation
Topic: Basic Science » Basic Science » 08 - Innate Immunity; Chemokines, Cytokines, Complement
Session Information
Session Name: Innate Immunity; Chemokines, Cytokines, Complement
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Hall C
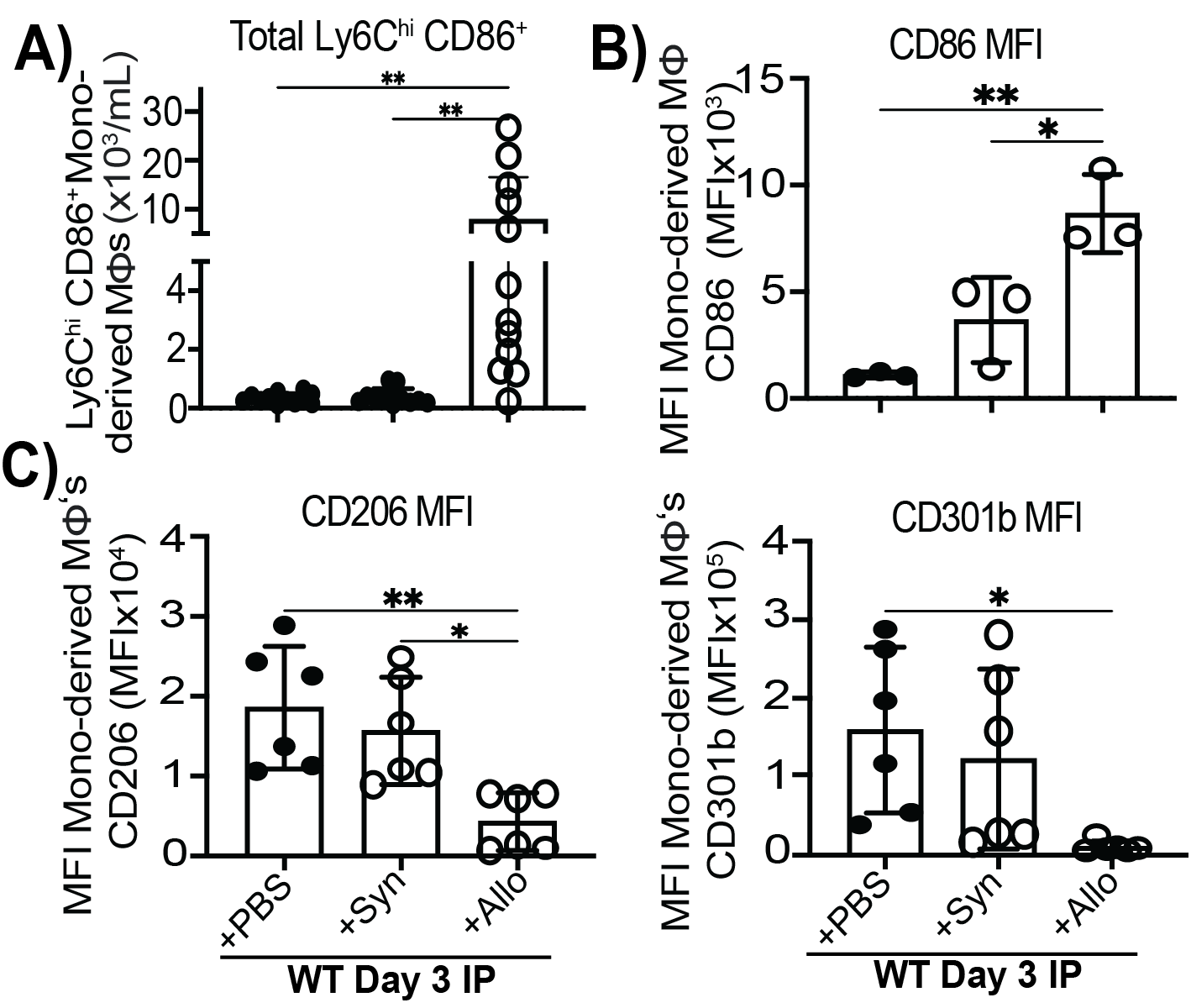
*Purpose: Monocytes differentiate into macrophage subsets that orchestrate tissue repair in response to damage signals and local cytokines. After heart transplant (Tx), graft IL-33 acts as a reparative/regulatory damage-associated molecular pattern (rDAMP) that metabolically reprograms macrophages to enable their differentiation into reparative subsets that protects against chronic Tx rejection. Yet, innate allorecognition of MHCI (H2-Dd) by paired immunoglobulin-like receptor (PIR)-A3 on monocytes generates pro-inflammatory monocyte-derived dendritic cells that promotes BALB/c kidney and heart Tx rejection. PIR-A responses are thought to be negatively regulated by co-expressed PIR-B binding to self MHCI. How PIRs shape macrophage differentiation from monocytes after Tx is poorly understood. Thus, we hypothesize that recognition of self versus allogeneic MHCI by PIRs modulates rDAMP-mediated macrophage differentiation into reparative macrophages following Tx.
*Methods: Macrophage differentiation in response to donor materials was tested by administration of 20×106 irradiated BALB/c allogeneic (H-2d; Allo) or B6 syngeneic (H-2b; Syn) splenocytes intraperitoneally and/or with recombinant IL-33. Flow cytometry of peritoneal leukocytes was utilized to assess macrophage differentiation in wildtype (WT), Rag2-/-γc-/-, Pira-/- and Pirb-/- B6 mice.
*Results: Alloantigen transformed local macrophages by 3 days post donor cell delivery. WT and Rag2-/-γc-/- mice had profound increases in monocyte-derived inflammatory Ly6chi CD86hi macrophages compared to syn-treated mice (Fig. 1a,b). This shift was at the expense of CD206+ CD301+ macrophages, which dominated after PBS or syn exposure (Fig. 1c). Reparative macrophage loss was rescued by co-administering IL-33. Interestingly, Pirb-/- mice had greatly reduced C206+ CD301+ reparative macrophages and decreased expression of the IL-33 receptor, Stimulation-2 (ST2).
*Conclusions: These data indicate allorecognition by monocytes or macrophages can disrupt the typical generation of reparative macrophages in response to injured self. We also show that monocyte-derived macrophage PIR-B recognition of self-MHCI may trigger ST2 expression needed for IL-33-stimulated differentiation into reparative macrophages. These new data provide important insights into how innate allorecognition and repair pathways intersect to direct the differentiation of reparative or inflammatory macrophages after Tx.
To cite this abstract in AMA style:
Warunek J, Lucas A, Mathews LR, Oberbarnscheidt M, Lakkis F, Turnquist H. Alloimmune Recognition Blocks Recipient Macrophage Differentiation into Reparative Subsets [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/alloimmune-recognition-blocks-recipient-macrophage-differentiation-into-reparative-subsets/. Accessed June 30, 2025.« Back to 2022 American Transplant Congress