Acute Antibody Mediated Rejection Treatment Impact on Class I and Class II Anti-HLA Antibodies in Pediatric Kidney Transplant Recipients
1University Health System, San Antonio, TX
2College of Pharmacy, Pharmacotherapy Division, The University of Texas at Austin, Austin, TX
3UT Health San Antonio, San Antonio, TX.
Meeting: 2018 American Transplant Congress
Abstract number: B219
Keywords: Histocompatibility, HLA antibodies, IVIG, Plasmapheresis
Session Information
Session Name: Poster Session B: Kidney: Pediatrics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Purpose: Characterize class I and class II donor specific antibodies (DSA) response to acute antibody mediated rejection (AMR) treatment in pediatric kidney transplant recipients (KTR).
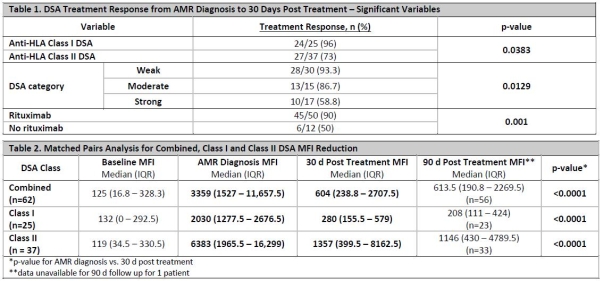
Methods: A single-center retrospective chart review of pediatric KTR receiving a renal transplant between 5/1/13 to 9/30/17 was conducted. Patients < 18 years old at transplant experiencing first episode of acute AMR and received treatment were included. DSA were identified by single antigen bead Luminex® assays at: time of transplant, acute AMR diagnosis, ~30 days post treatment, and ~90 days post treatment. DSA categorized as weak: 1000 – 2999 mean fluorescence intensity (MFI); moderate: 3000 – 9999; strong: > 10,000. Treatment response was defined as MFI decrease ≥30% from diagnosis to ~30 days post treatment.
Results: 62 DSA were identified from 12 patients [25 (40%) Class I and 37 (60%) Class II]. 100% of DSA were treated with IVIG and PP. 50 (80%) received rituximab. The same 51/62 (82%) DSA achieving > 30% MFI reduction also achieved a categorical shift. Multivariate analysis revealed rituximab (p=0.0041) and lower MFI (p=0.0017) at diagnosis as independent predictors of treatment response. Univariate analysis reported in Table 1. Diagnosis by protocol biopsy and documented non-adherence at diagnosis were not predictors of treatment response. Matched pairs analysis for DSA MFI reduction reported in Table 2.
Conclusion: Rituximab and lower MFI at AMR diagnosis were positive predictors of DSA MFI reduction. Treatment resulted in a significant reduction in MFI from AMR diagnosis to 30 days post treatment, without DSA MFI rebound at 90 days. The same DSA achieved > 30% and categorical shift, indicating > 30% is an appropriate AMR treatment target. Class I DSA were more likely to respond to treatment. As DSA with higher MFIs were less likely to respond to treatment, a more timely AMR diagnosis could lead to an improved response.
CITATION INFORMATION: Kincaide E., Hitchman K., Hall R., Yamaguchi I., Crowther B. Acute Antibody Mediated Rejection Treatment Impact on Class I and Class II Anti-HLA Antibodies in Pediatric Kidney Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Kincaide E, Hitchman K, Hall R, Yamaguchi I, Crowther B. Acute Antibody Mediated Rejection Treatment Impact on Class I and Class II Anti-HLA Antibodies in Pediatric Kidney Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/acute-antibody-mediated-rejection-treatment-impact-on-class-i-and-class-ii-anti-hla-antibodies-in-pediatric-kidney-transplant-recipients/. Accessed March 2, 2026.« Back to 2018 American Transplant Congress