A Two-Step Cell-Based Assay Predicts Immunosuppression-Related Adverse Events in Kidney Transplant Recipients
Transplantation Unit, Renal Division, Medecine Department, Laval University, Québec, QC, Canada
Meeting: 2020 American Transplant Congress
Abstract number: A-308
Keywords: B cells, Immunosuppression, Kidney transplantation, Tumor necrosis factor (TNF)
Session Information
Session Name: Poster Session A: Biomarkers, Immune Assessment and Clinical Outcomes
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
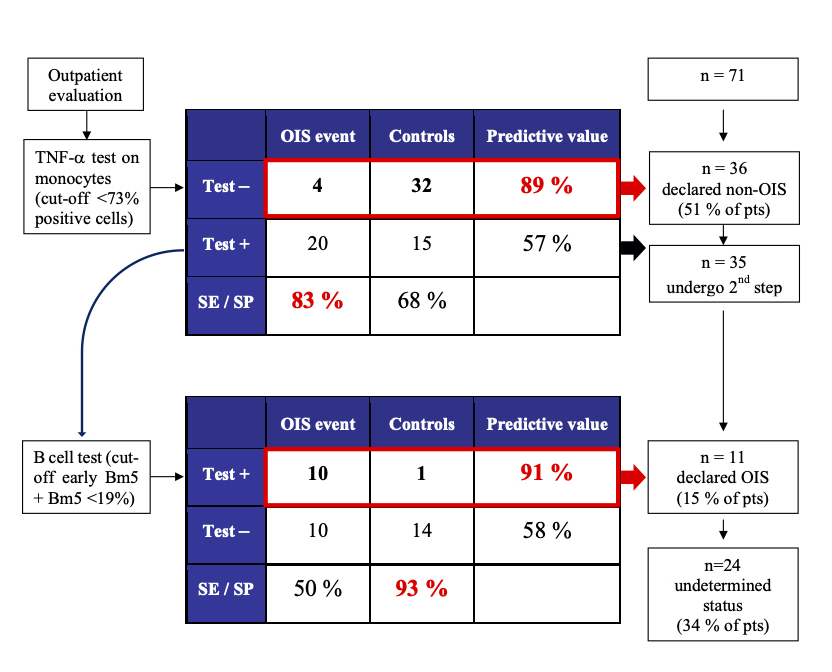
*Purpose: No clinical tool is available to dynamically access immunosuppression status, which results in serious, often life-threatening adverse effects of immunosuppressants. We recently validated that measurement of TNF-a production by CD14+16+ intermediate monocytes following stimulation by EBV peptides has high sensitivity but low specificity for detecting over-immunosuppression (OIS) events. Optimization of this cell-based assay was mandatory to increase its clinical value.
*Methods: Peripheral blood mononuclear cells from 71 kidney recipients were harvested to develop and validate a second step with high specificity. Patients were classified as cases or controls according to the occurrence of opportunistic infection, recurring bacterial infections, or de novo neoplasia in the 12 months following blood collection. Patients who tested positive in the first step of the assay were randomly allocated to a discovery and validation set.
*Results: A discovery phase focusing on mature B (Bm) cells showed that a percentage of less than 19% of circulating early Bm5 + Bm5 cells had a specificity of 90% for over-immunosuppression. The validation set revealed a specificity of 100% while bootstrapping analysis estimated specificity of 93%. In multivariable proportional hazards analysis adjusted for age, eGFR, time post transplant and immunosuppression, the risk of OIS events in patients who were classified positive was more than eight times higher than patients who tested negative (adjusted HR = 8.9; 95% CI 1.9-42.23; p = 0.006). In all, the two-step assay classified two-thirds of the patients of this cohort as over-immunosuppressed or controls, with negative and positive values of 89% and 91% respectively.
*Conclusions: This cell-based approach seems useful for identifying OIS kidney recipients. A multicenter validation is underway.
To cite this abstract in AMA style:
Bouchard-Boivin F, Désy O, Béland S, Serres SDe. A Two-Step Cell-Based Assay Predicts Immunosuppression-Related Adverse Events in Kidney Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/a-two-step-cell-based-assay-predicts-immunosuppression-related-adverse-events-in-kidney-transplant-recipients/. Accessed July 5, 2025.« Back to 2020 American Transplant Congress