A Prospective Pilot Study Evaluating the Safety and Efficacy of Everolimus for the Prevention of CMV and BK Viral Infection (BKV) in Broadly Sensitized Kidney Transplant Recipients Following Desensitization With IVIG and Rituximab: Interim Analysis
1Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA
2Transplant Immunology Lab, Cedars-Sinai Medical Center, Los Angeles, CA.
Meeting: 2015 American Transplant Congress
Abstract number: A79
Keywords: Cytomeglovirus, Infection, Polyma virus, Sensitization
Session Information
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Exhibit Hall E
Introduction: Everolimus, a second generation mTOR inhibitor, may prevent CMV and BKV infection after kidney transplantation. Broadly sensitized patients are at greater risk of viral infection due to increased immunosuppression requirements. Here we report interim results of a prospective pilot study exploring the safety and efficacy of everolimus with low-exposure tacrolimus for the prevention of viral infection and sensitization in this high risk population.
Methods: Broadly sensitized patients (PRA>30%) desensitized with IVIG and rituximab were eligible to participate. All received alemtuzumab induction and were maintained on everolimus (target trough 3-8), tacrolimus (target 4-7 stepped down to 2-5 after month six), and prednisone. CMV and BKV pcr's were obtained at regular intervals. Protocol biopsies were done at time 0 and six months. Outcomes were compared to a retrospective, concurrent cohort of sensitized patients maintained on tacrolimus, mycophenolate, and prednisone.
Results: Eighteen patients have been enrolled in the study thus far. One patient was excluded due to a protocol deviation. Results are shown below for a median follow-up of six months.
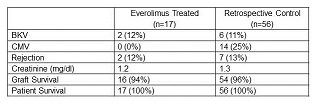
There was one graft loss due to FSGS in the study group and 2 in the control group (FSGS and thrombosis). There were a total of 12 adverse events in seven patients in the study group. Six patients developed proteinuria, two of which required discontinuing therapy. There was no development of de novo or rebound DSA.
Conclusion: Early results indicate that the use of everolimus in high immunologic risk patients is safe and effective. CMV was significantly reduced in patients treated with everolimus, however the rates of BKV were similar. Rejection rates, allograft function, patient survival and graft survival were similar to the concurrent control group.
To cite this abstract in AMA style:
Kahwaji J, Louie S, Vo A, Choi J, Toyoda M, Ge S, Wongsaroj P, Peng A, Villicana R, Jordan S. A Prospective Pilot Study Evaluating the Safety and Efficacy of Everolimus for the Prevention of CMV and BK Viral Infection (BKV) in Broadly Sensitized Kidney Transplant Recipients Following Desensitization With IVIG and Rituximab: Interim Analysis [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/a-prospective-pilot-study-evaluating-the-safety-and-efficacy-of-everolimus-for-the-prevention-of-cmv-and-bk-viral-infection-bkv-in-broadly-sensitized-kidney-transplant-recipients-following-desensiti/. Accessed February 16, 2026.« Back to 2015 American Transplant Congress
