Eculizumab in the Treatment of Early Severe Acute Antibody Mediated Rejection Following HLA-Compatible Kidney Transplantation-Case Series
1Transplant Nephrology, UCSF Medical Center, San Francisco, CA
2Nephrology, Hospital Gregorio Maranon, Madrid, Spain.
Meeting: 2015 American Transplant Congress
Abstract number: A132
Keywords: Graft function, Kidney transplantation, Monoclonal antibodies, Rejection
Session Information
Session Name: Poster Session A: Kidney Antibody Mediated Rejection
Session Type: Poster Session
Date: Saturday, May 2, 2015
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Exhibit Hall E
Severe early acute antibody-mediated rejection (AMR) is associated with poor allograft outcomes. Eculizumab is a humanized mouse monoclonal antibody against complement factor C5 that has been successfully used in the field of transplantation to prevent recurrent atypical hemolytic uremic syndrome (aHUS) and more recently in antibody mediated rejection. Here, we report 5 cases of severe acute AMR within 2 weeks post transplant (4 kidney alone, one after simultaneous kidney-pancreas). Three of them had histologic evidence of thrombotic microangiopathy and two with failure to respond to the standard treatment who were successfully treated with 2-4 doses of eculizumab in addition to plasmapheresis, intravenous immunoglobulins (IVIG) +/- Rituximab. Two patients who were ABO incompatible underwent desensitization, and two were highly sensitized with calculated panel reactive antibody (cPRA) above 80%. All patients had negative flow cross-match and no anti-HLA donor specific antibodies pre-transplant. All patients received antithymocyte globulin induction therapy followed by standard maintenance immunosuppression with tacrolimus, mycophenolate mofetil/sodium, and prednisone. All patients responded within 2-3 days after eculizumab administration with improved allograft function. Three patients underwent 6 month protocol biopsy showing resolution of anti-allograft activity with no evidence of transplant glomerulopathy. At follow-up (9-23 months), all patients had a functioning allograft with mean serum creatinine 2.08 (1.17-4.75) mg/dl at 1 month, 1.15 (0.83-1.93) mg/dl at 3 months and 1.13 (1.06-1.73) mg/dl at 9-12 months.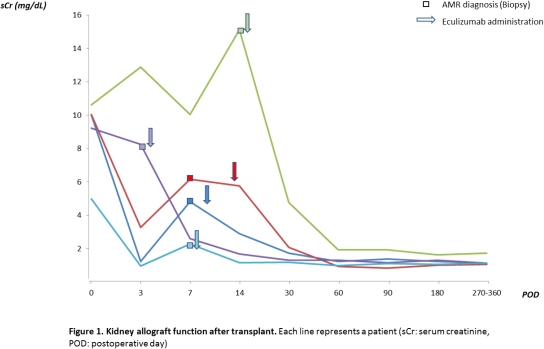 Our case series emphasizes the fact that eculizumab seems to be an effective therapy when used early in patients with severe acute AMR by inhibiting complement related graft injury without an apparent increased risk of infectious complications. Further studies are needed to establish the dosing and duration of eculizumab and to explore its cost effectiveness compared to other treatment modalities.
Our case series emphasizes the fact that eculizumab seems to be an effective therapy when used early in patients with severe acute AMR by inhibiting complement related graft injury without an apparent increased risk of infectious complications. Further studies are needed to establish the dosing and duration of eculizumab and to explore its cost effectiveness compared to other treatment modalities.
To cite this abstract in AMA style:
Singh M, Macias N, Tatapudi V, Lee B. Eculizumab in the Treatment of Early Severe Acute Antibody Mediated Rejection Following HLA-Compatible Kidney Transplantation-Case Series [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/eculizumab-in-the-treatment-of-early-severe-acute-antibody-mediated-rejection-following-hla-compatible-kidney-transplantation-case-series/. Accessed January 10, 2026.« Back to 2015 American Transplant Congress
