Alloreactive Human Regulatory T Cell-Derived Extracellular Vesicles Exhibit Adenosinergic Mediated Suppression and Potential for Infectious Tolerance
Brigham and Women's Hospital, Boston, MA
Meeting: 2022 American Transplant Congress
Abstract number: 588
Keywords: Kidney transplantation, T cells, Tolerance
Topic: Basic Science » Basic Science » 10 - Treg/Other Regulatory Cell/Tolerance
Session Information
Session Name: Tregs and Tolerance
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:50pm-7:00pm
Presentation Time: 6:50pm-7:00pm
Location: Hynes Room 304 / 306
*Purpose: Regulatory T cells (Tregs) suppress the immune response through both contact dependent and independent mechanisms. Extracellular vesicles (EVs) are emerging as novel contact independent regulatory mediators employed by Tregs, however their suppressive function has not been well described in humans. Our purpose was to quantify the suppressive potency of human Treg-derived EVs using donor alloreactive Treg-enriched cell lines (ASTRLs) and to identify mechanisms by which they exert suppression.
*Methods: Culture supernatant was collected from ASTRL cell lines under allopeptide stimulation and rest conditions. Large and small EV fractions were isolated via differential ultracentrifugation at 10,000xg x30 min (lgEV) and 100,000xg x 2 hr (smEV) before characterization by nanoparticle tracking analysis and electron microscopy. Suppression assays were performed to assess additive suppressive potency of ASTRL-EVs. EV extracellular ATP hydrolytic capacity was assessed in the presence and absence of CD39 inhibition. Cell proliferation and cytokine production of EV-treated PBMCs were also analyzed to assess EV impact on recipient cells.
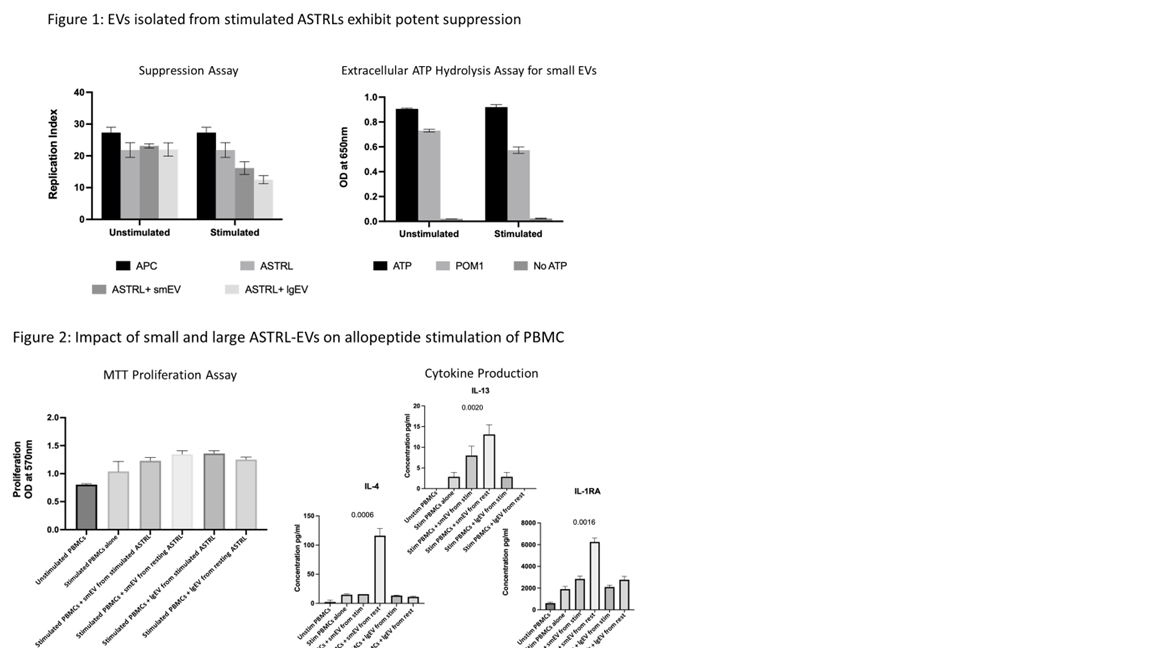
*Results: Both small and large EVs isolated from stimulated ASTRLs increased the suppressive ability of ASTRLs in an in vitro suppression assay (Fig. 1). Small EVs isolated from both stimulated and rest conditions were able to hydrolyze extracellular ATP independently, an effect which was more pronounced in stimulated EVs and inhibited by CD39 inhibitor POM1 (Fig. 1). This suggests that functional CD39 is expressed on smEVs and that smEVs are preferentially loaded with CD39 upon stimulation. Interestingly, there was no effect of ASTRL-EV treatment on the proliferative response of PBMCs to allopeptide stimulation (Fig. 2). However, the cytokine production was significantly biased towards a regulatory environment in the presence of smEVs released from rest where IL-13, IL-4 and IL-1RA production was significantly increased (Fig. 2).
*Conclusions: ASTRL-EVs released upon allopeptide stimulation enhance the suppressive capacity of ASTRLs and exhibit an increase in CD39 function. Additionally, smEVs released under rest conditions appear to modulate cytokine production of recipient cells towards a regulatory microenvironment. This data suggests ASTRL-EVs may facilitate infectious tolerance to allopeptide and could be useful adjuncts to autologous regulatory cell therapy. However, more thorough investigation is needed to better understand distinct large and small EV cargo and the biologic mechanisms they employ.
To cite this abstract in AMA style:
Schreiber B, Tripathi S, Chandraker A. Alloreactive Human Regulatory T Cell-Derived Extracellular Vesicles Exhibit Adenosinergic Mediated Suppression and Potential for Infectious Tolerance [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/alloreactive-human-regulatory-t-cell-derived-extracellular-vesicles-exhibit-adenosinergic-mediated-suppression-and-potential-for-infectious-tolerance/. Accessed February 14, 2026.« Back to 2022 American Transplant Congress