Local Intragraft Humoral Immune Response in Chronic Lung Allograft Dysfunction
1Tenri Hospital Japan, University Health Network Canada, Nara, Japan, 2University Health Network, Toronto, ON, Canada, 3Multi-Organ Transplantation, University Health Network, Toronto, ON, Canada, 4Sunnybrook Health Science Centre, Toronto, ON, Canada
Meeting: 2022 American Transplant Congress
Abstract number: 1492
Keywords: Autoimmunity, B cells, HLA antibodies, Rejection
Topic: Clinical Science » Lung » 64 - Lung: All Topics
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Hall C
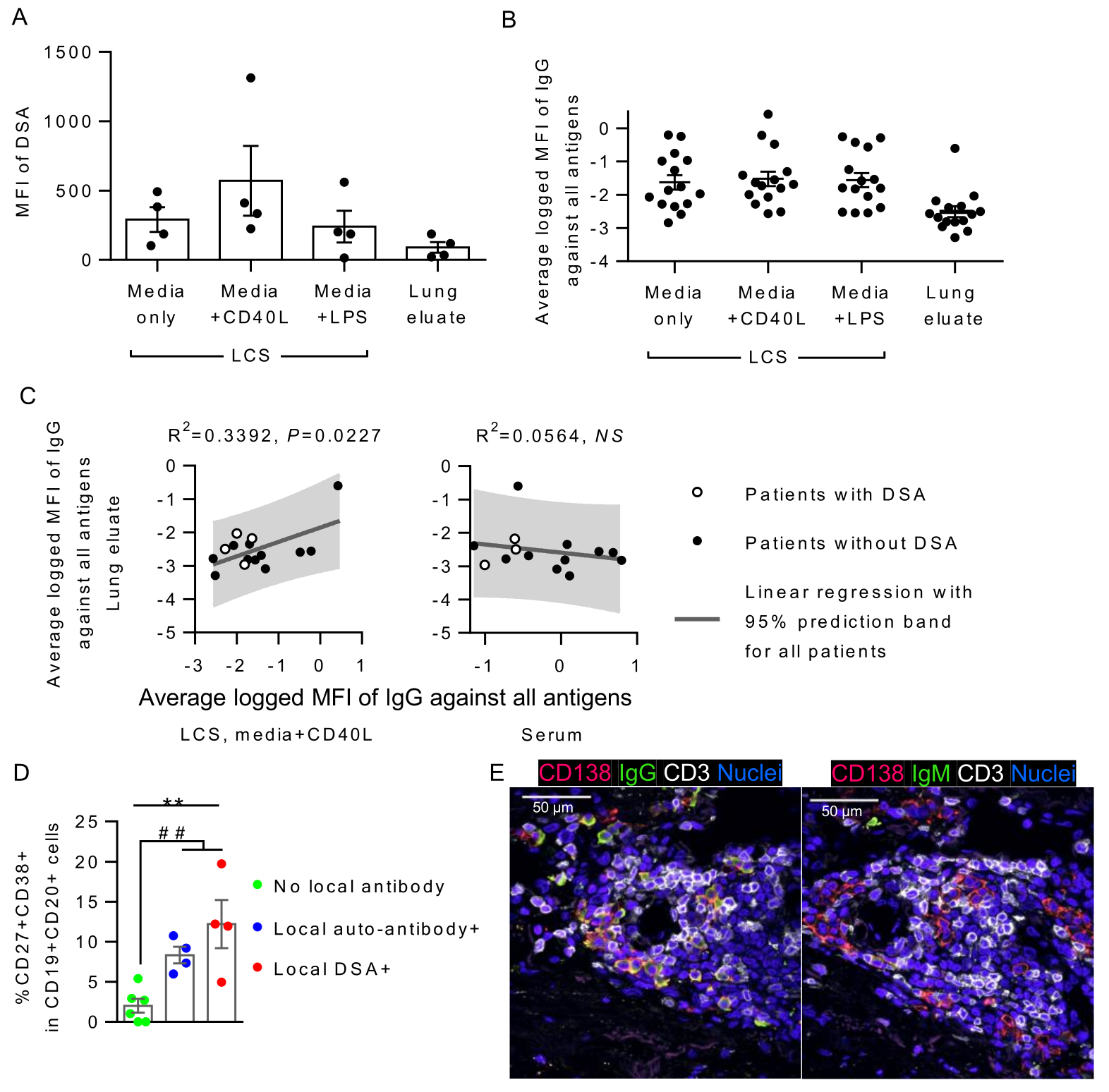
*Purpose: Donor HLA-specific antibody (DSA) and antibody against self-antigens (self-antigen antibody) can cause injury and lead to chronic lung allograft dysfunction (CLAD) after lung transplantation (LTx). Despite increasing interest in the role of antibodies in lung transplantation, it remains uncertain whether these are produced locally in the graft or primarily remain in the circulation. We aimed to determine whether DSA and antibodies directed at self antigens are produced in CLAD lungs.
*Methods: Fresh whole lungs were prospectively collected from 15 LTx patients who underwent re-transplant or medically assisted death due to CLAD. 0.3g of fresh lung tissue was cultured for 4 days with or without lipopolysaccharide or CD40L. Lung culture supernatant (LCS) was sampled. Lung eluate was obtained from 0.3g of frozen lung tissue. Lung B and plasma cells were analyzed by flow cytometry. Mean fluorescent intensity (MFI) of DSA and self-antigen antibodies were measured by Luminex and antigen microarray, respectively. Lymphoid aggregates (LAs) in the lung graft were assessed by hematoxylin-eosin staining and immunofluorescence.
*Results: CD40L-stimulated LCS from all 4 patients who had serum DSA at lung isolation were positive for DSA, while the matched lung eluate were positive for DSA only in 3 of those patients. (Fig. A). Antibodies against self-antigens were also detectable in LCS and lung eluates (Fig. B). MFIs of IgG against self-antigens of lung eluates correlated with those of CD40L-stimulated LCS but not those of other LCS or serum (Fig. C). Patients whose CD40L-stimulated LCS was positive for DSA or self-antigen antibody (local antibody) showed significantly higher proportions of activated (CD27+CD38+, AID+, or CD86+) B cells and plasma cells in the lung tissue compared to those who had no local antibody in LCS (Fig. D). LAs of patients with local antibody production were surrounded by significantly higher numbers of IgG+ and IgM+ plasma cells (Fig. E), with larger IL-21+ fractions.
*Conclusions: Donor lung graft itself is capable of producing DSA and self-reactive antibodies. Further investigation is required to demonstrate the clinical relevance of locally produced antibodies in the development of CLAD.
To cite this abstract in AMA style:
Miyamoto E, Juvet SC, Vosoughi D, Wang J, Al-Refaee J, Daigneault T, Duong A, Moshkelgosha S, Keshavjee S, Tinckam K, Chruscinski A, Hwang D, Martinu T. Local Intragraft Humoral Immune Response in Chronic Lung Allograft Dysfunction [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/local-intragraft-humoral-immune-response-in-chronic-lung-allograft-dysfunction/. Accessed March 7, 2026.« Back to 2022 American Transplant Congress