Clinical Significance of Delayed Graft Function and Slow Graft Function in Living Donor Kidney Transplantation
1Seoul National University Hospital, Seoul, Korea, Republic of, 2Boramae Medical Center, Seoul, Korea, Republic of, 3Seoul National University Bundang Hospital, Seongnam-si, Korea, Republic of
Meeting: 2022 American Transplant Congress
Abstract number: 1066
Keywords: Graft failure, Graft function, Graft survival, Kidney transplantation
Topic: Clinical Science » Kidney » 44 - Kidney Acute Antibody Mediated Rejection
Session Information
Session Name: Kidney Acute Antibody Mediated Rejection
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The delayed graft function (DGF) after kidney transplantation (KT) affects long-term graft and patient survival. We explored the time-trend of DGF and associated risk factors in the recent era in which incompatible living donor KTs are increasing. In addition, and the impact of DGF on graft and patient outcomes was compared according to the donor types.
*Methods: We reviewed electrical medical records of KT recipients from 2010 to 2021 in three referral hospitals. Patients were divided into three groups according to the degree of kidney function after KT as follows; DGF was defined as the need for dialysis, slow graft function (SGF) as serum creatinine levels ≥2.0 mg/dL but no need for dialysis, and immediate graft function (IGF) as serum creatinine levels <2.0 mg/dL within 1-week after KT. Risk factors for the development of DGF were investigated. After then, an impact of DGF on death-censored graft failure (DCGF) and all-cause mortality were explored.
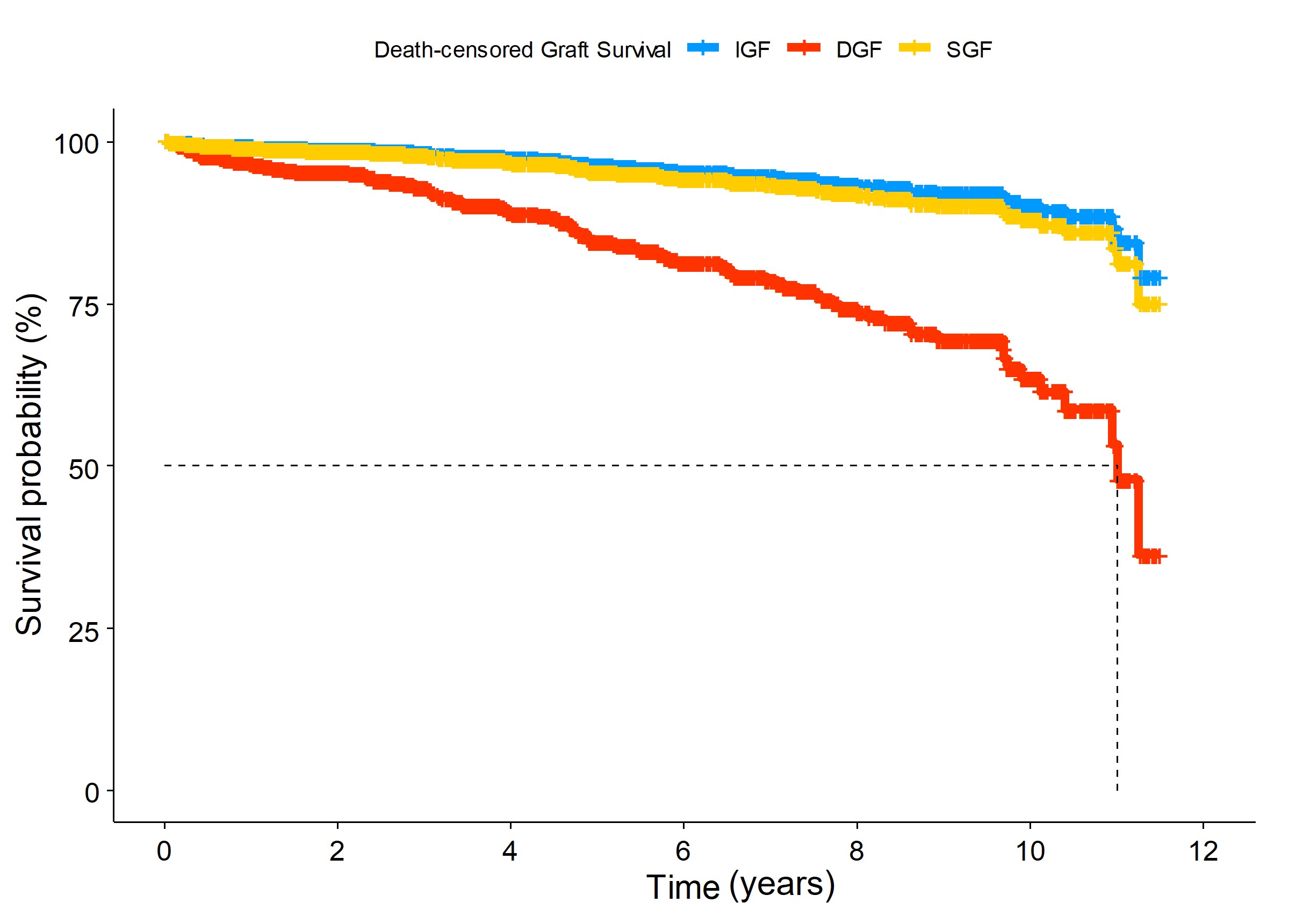
*Results: Of 2,304 kidney transplants, 102 (4.4%) recipients experienced DGF and 162 (7.0%) cases of SGF occurred. DGF was found more in deceased donor (10.5%) than living donor (1.3%) KT. Interestingly, about three fourths of DGF were developed since 2016 whereas SGF was reduced less than half in the same period. DGF tended to occur in recipients having old age, diabetic end-stage kidney disease, longer dialysis duration, allograft from deceased or older donor, and a greater number of HLA mismatches. In multivariate Cox regression model, DGF elevated DCGF risk as 4.34 (Figure 1. 95% CI=2.29-8.24, p<0.001) although SGF did not have impact on the graft failure. During an average of 4.63 years, DCGF occurred in 104 patients and 105 patients died. DGF was an independent risk factor for both DCGF and patient death (adjusted HR, 2.86; 95% CI, 1.57-5.23, p<0.001), respectively. In addition, in living donor KT, DGF increased DCGF risk as 10.27 and one patient with DGF died. DGF of deceased donor KT was associated with a 2.79-fold increase in DCGF and a 2.34-fold increase in all-cause mortality. The most common cause of DGF in living donor KT was acute rejection including T-cell-mediated (n=5) and active antibody-mediated (n=5) rejection.
*Conclusions: In this study, we found that DGF tended to increase in recent years. DGF was associated with worse graft and patient outcomes. Especially, DGF showed much worse graft outcomes in living donor KT, although its absolute incidence was rare.
To cite this abstract in AMA style:
Song J, Jeong S, Min S, Ha J, Lee J, Jeong J, Kim Y, Kim Y, Lee H. Clinical Significance of Delayed Graft Function and Slow Graft Function in Living Donor Kidney Transplantation [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-significance-of-delayed-graft-function-and-slow-graft-function-in-living-donor-kidney-transplantation/. Accessed March 5, 2026.« Back to 2022 American Transplant Congress