Thymoglobulin Induction Protocol: Quality Improvement Project via Clinical Audit
1University of Virginia Health System, Charlottesville, VA, 2Nephrology, University of Virginia, Charlottesville, VA, 3University of Virginia, Charlottesville, VA
Meeting: 2022 American Transplant Congress
Abstract number: 608
Keywords: Antilymphocyte antibodies, Dosage, Immunosuppression, Induction therapy
Topic: Administrative » Administrative » 01 - Quality Assurance Process Improvement & Regulatory Issues
Session Information
Session Name: Quality Assurance Process Improvement & Regulatory Issues
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: The aim of our study was to analyze the adequacy of the thymoglobulin (rATG) induction protocol for the expanding and higher complexity kidney transplant (KT) population being performed at our center and propose evidence based updates.
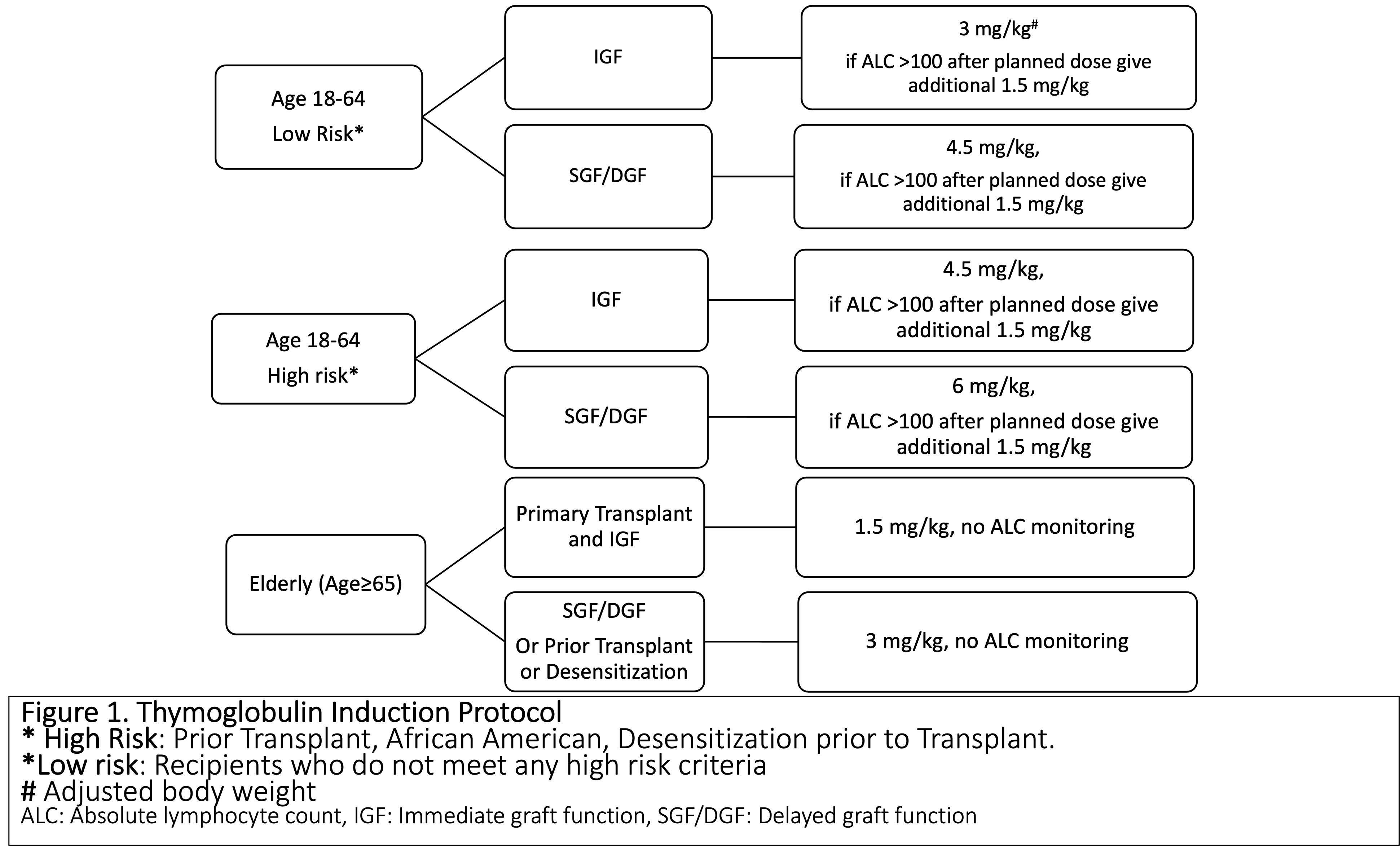
*Methods: A single center, prospective data collection for KT performed between February and July 2021 was conducted. Treating physicians were advised on recommended rATG dose based on the risk-stratification as per the induction protocol (Fig 1), with final dose determined by physician. Reasons for dose modification were captured and analyzed, followed by recommendations on updating protocol based on literature review.
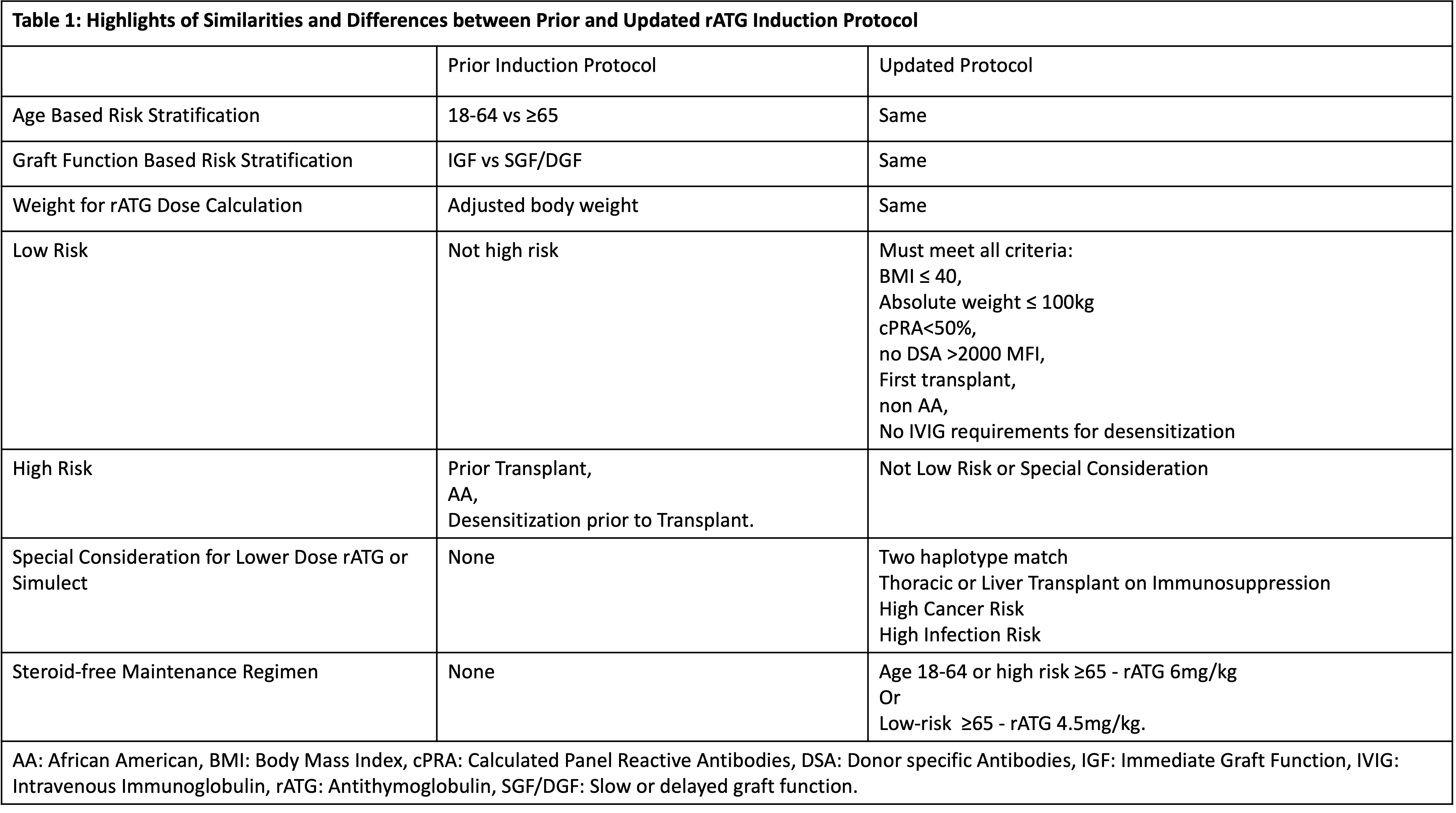
*Results: 59 KT, with mean age of 52 years, 54% Caucasian and 33% African Americans (AA) ,15% prior transplants, 68% deceased donor, were studied. According to the risk stratification: 44% were low risk, 37% were high risk, 19% were elderly.The protocol deviation was high (39%), with the majority receiving higher rATG dose (n=19) due to pre-existing donor specific antibodies (DSA) and panel reactive antibody (PRA) (n=6), high BMI (n=6), inadequate lymphocyte depletion, steroid-withdrawal regimen, and perceived low total rATG dose (adjusted body weight (BW) used rather than actual BW). However, these dose modifications were not uniformly applied, with no statistical difference in rATG doses based on BMI, age, DSA, or PRA. The results were presented to transplant team members along with the literature review on key topics (BMI, AA, PRA/DSA, lymphocyte depletion, appropriate weight for rATG dose, steroid-free regimens) and suggestions of protocol modification. After deliberations, the protocol has been updated with goal to decrease deviations by 50%, monitored by prospective clinical audit (Table 1).
*Conclusions: Utilizing clinical audit to quantify and qualify the performance shortfalls of rATG induction protocol, we successfully implemented system based protocol modifications to reflect the changing needs of the KT program and prevailing standard of care. We propose that clinical audit of protocols in the KT is a valuable tool for quality improvement and enhances patient care.
To cite this abstract in AMA style:
Mouawad S, Dann J, Kumar A, Rao S. Thymoglobulin Induction Protocol: Quality Improvement Project via Clinical Audit [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/thymoglobulin-induction-protocol-quality-improvement-project-via-clinical-audit/. Accessed January 26, 2026.« Back to 2022 American Transplant Congress