Bone Marrow Plasma Cell Heterogeneity Drives Resistance of Proteasome Inhibitor Therapy
1Immunobiology, Cincinnati Children's Hospital, Cincinnati, OH, 2Surgery, University of Cincinnati College of Medicine, Cincinnati, OH, 3Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, OH, 4Biomedical Informatics, Cincinnati Children's Hospital, Cincinnati, OH
Meeting: 2020 American Transplant Congress
Abstract number: D-095
Keywords: B cells, HLA antibodies, Kidney transplantation, Sensitization
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Desensitization
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Patients sensitized to HLA antigens by prior transplantation, pregnancy or blood transfusion may have markedly prolonged waiting times for transplant. Moreover, once transplanted, the development of HLA antibodies (Abs) represent the primary cause of renal allograft loss. The proteasome inhibitors (PIs) carfilzomib and bortezomib target HLA Ab-producing bone marrow plasma cells (BMPCs) and reduce HLA Ab levels. However, the effects of PIs are suboptimal, often transient, and limited due to de novo or treatment-induced drug resistance that emerges through mechanisms that remain elusive. One potential explanation for plasma cell (PC) resistance is their residence within the BMPC niche, which current dogma suggests is orchestrated by the chemokine receptor CXCR4. Here, we evaluated the impact of bortezomib therapy after treatment with CXCR4/CXCL12 blocker (plerixafor) on PC populations in the blood and the bone marrow (BM).
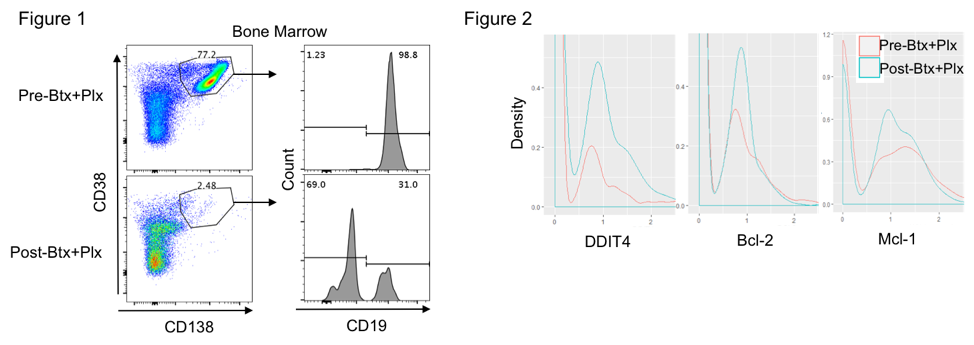
*Methods: Peripheral blood and BM aspirates were collected before and after treatment with plerixafor +/- bortezomib from HLA-sensitized kidney transplant candidates. We performed a temporal assessment of anti-HLA-Abs by single antigen-bead assays, and PC mobilization to the blood and homeostasis within the BM were assessed by flow cytometry. After PBMC isolation, we characterized the CD138+CD38++IgG–CD27+PCs as newly formed plasma cells (NFPCs, CD19+) and long-lived plasma cells (LLPCs, CD19–). In addition, heterogeneity and transcriptomic analysis of individual CD138+ PCs from the BM were assessed before and after treatment by single cell RNA sequencing.
*Results: We observed that plerixafor preferentially mobilized CD19+ NFPCs into the peripheral blood. After bortezomib treatment, there was a substantial loss of NFPCs in the BM, while CD19–LLPCs were largely unaffected (Figure 1). We also observed transient loss of anti-HLA Abs as well as an upregulation of hypoxia-inducible factors (HIF)-signaling targets and apoptotic effectors in PCs following PI and plerixafor therapy (Figure 2).
*Conclusions: Our data show that plerixafor and bortezomib treatment has a preferential effect on NFPCs in the BM and that cells with hypoxic/HIF-1αsignature and low apoptotic potential are enriched in surviving cells.
To cite this abstract in AMA style:
Castro-Rojas CM, Tremblay S, Driscoll J, Roskin K, Alloway RR, Woodle E, Hildeman DA. Bone Marrow Plasma Cell Heterogeneity Drives Resistance of Proteasome Inhibitor Therapy [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/bone-marrow-plasma-cell-heterogeneity-drives-resistance-of-proteasome-inhibitor-therapy/. Accessed February 25, 2026.« Back to 2020 American Transplant Congress