Hyperspectral Imaging of Human Kidney Allografts
1Department of Visceral, Transplant-, Thoracic and Vascular Surgery, University Clinic Leipzig, Leipzig, Germany, 2Innovation Center for Computer Assisted Surgery, University Clinic Leipzig, Leipzig, Germany
Meeting: 2020 American Transplant Congress
Abstract number: C-110
Keywords: Graft failure, Graft function, Image analysis, Kidney
Session Information
Session Name: Poster Session C: Kidney Technical
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Hyperspectral imaging (HSI) is capable to deliver non-contact, non-ionizing and non-invasive quantitative diagnostic information about tissue pathology, morphology and composition, based on the spectral characteristics of the investigated tissue. Since tools for objective intraoperative graft viability and performance assessment are lacking, we applied this novel technique to human kidney transplantation.
*Methods: Hyperspectral images of distinct components of kidney allografts (blood vessels, parenchyma, ureter) were taken at different timepoints before and after reperfusion. Images were analyzed using specialized HSI acquisition software capable to compute oxygen saturation levels (StO2), near infrared perfusion indices (NIR), organ hemoglobin indices (OHI) and tissue water indices (TWI) of explored tissues. Primary study endpoint was delayed graft function (DGF).
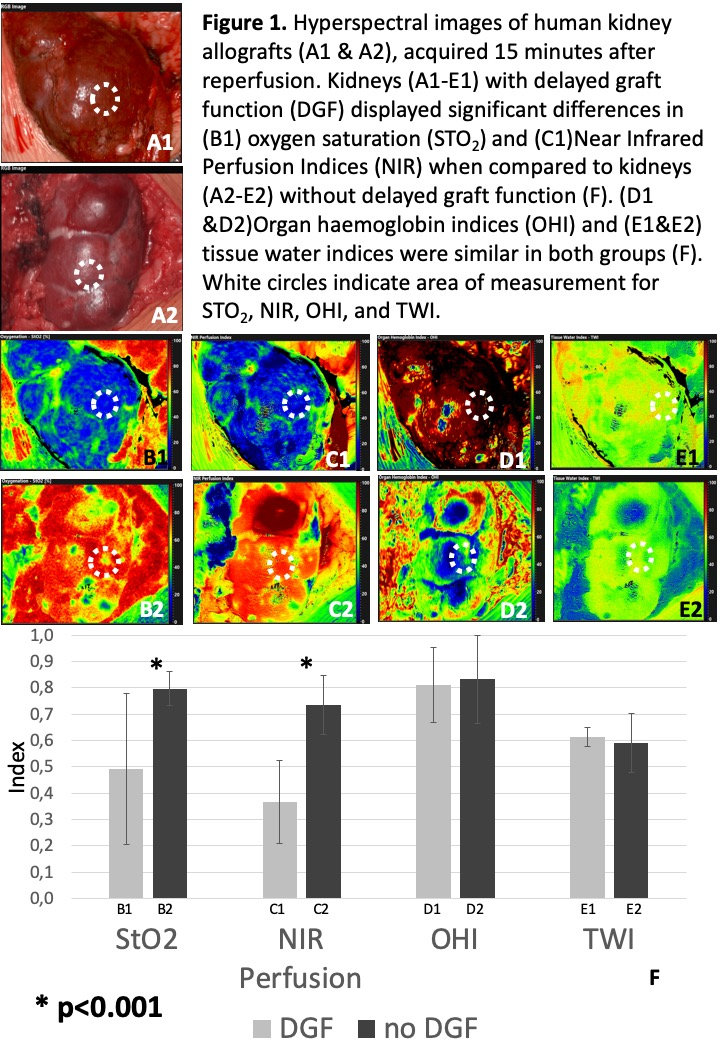
*Results: Seventeen kidney transplants were analyzed. Median recipient and donor age were 62 and 61 (range:2-74) years respectively. Cold ischemia time (CIT) was 10.8±4.1 hours and anastomosis time was 35±7 minutes (mean±SD). Two patients (11.8%) developed DGF. CIT was significantly increased (18.6±1.6) in patients with DGF (p<0.001). Kidneys with DGF furthermore displayed significant lower StO2 and NIR perfusion indices, 15 min after reperfusion (p<0.001, Figure 1.). HS images of the ureter displayed a significant decrease of NIR perfusion with increased distance to the renal pelvis, identifying well and poor perfused ureter segments for uretero-cystostomy (p<0.001). One pediatric patient (age: 2) developed early graft vein thrombosis detected by doppler ultrasound and HSI on POD1. Successful emergency thrombectomy could be intraoperatively visualized by HSI, providing real time information about tissue StO2 and NIR perfusion parameters, which dramatically decreased at time of thrombosis and regained normal values after thrombectomy (p<0.001).
*Conclusions: Intraoperative HSI is feasible and meaningful to predict DGF in renal allografts. Furthermore, it can be utilized for non-invasive image guided surgery, providing real time information about tissue oxygenation, perfusion, hemoglobin concentration and water concentration, hence allowing intraoperative viability assessment of the kidney parenchyma and the ureter.
To cite this abstract in AMA style:
Sucher R, Köhler H, Lederer A, Hau HM, Gockel I, Seehofer D. Hyperspectral Imaging of Human Kidney Allografts [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/hyperspectral-imaging-of-human-kidney-allografts/. Accessed January 7, 2026.« Back to 2020 American Transplant Congress