Novel Technique to Analyze Blood-Endothelial Cell Interactions Under Flow Conditions
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, 2University of Maryland, Baltimore, Baltimore, MD
Meeting: 2020 American Transplant Congress
Abstract number: C-381
Keywords: Adhesion molecules, Endothelial activation, Endothelial cells, Xenoreactive antibodies
Session Information
Session Name: Poster Session C: Endothelial Cell Biology
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
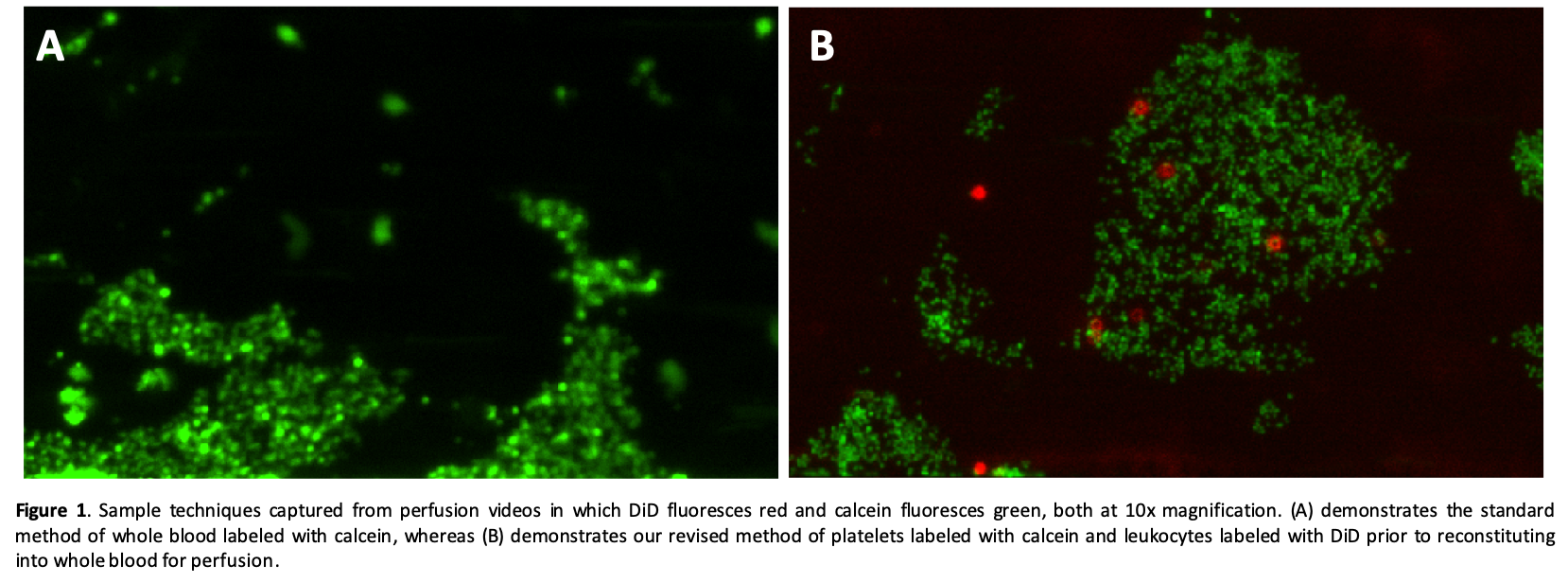
*Purpose: The Bioflux system has been used to study platelet-driven thrombosis and leukocyte adhesion. Typically, whole blood is stained with calcein (green) to evaluate platelet adhesion and aggregation but does not distinguish adherent leukocytes from larger platelet aggregates. We sought to develop a method to separately evaluate leukocyte (WBC) adhesion, which also take up calcein when whole blood is stained, while simultaneously measuring platelet aggregation during interactions between porcine endothelium and whole human blood.
*Methods: Porcine endothelia were cultured to confluence in microfluidic channels and then perfused with fluorescently stained human blood. Blood was separated into platelet-rich plasma (PRP), WBCs, and erythrocytes. In one condition, the 3 tubes were immediately recombined to reconstitute whole blood and then stained with calcein for 30 minutes (standard method). In the revised method, the PRP was stained with calcein and the WBCs were stained with Vybrant DiD for 30 minutes before recombining with erythrocytes as whole blood. The blood was then perfused at 5 dynes/cm2 and 37oC for one hour to mimic microcirculatory flow. Video images generated with the Montage software (Fluxion Biosciences) were overlaid to provide a visual representation of the staining.
*Results: Whole blood stained with calcein demonstrates heterogeneous green staining with a fine-grained speckled pattern (presumably reflecting individual adherent platelets) and larger particles reflecting adherent WBCs or platelet aggregates (Fig. 1A). When WBCs are separately labeled with DiD (red), the calcein-labeled platelet aggregates are readily distinguished from the adherent WBCs (Fig. 1B). These separate cell staining techniques are supported by preliminary flow cytometry analyses.
*Conclusions: Use of different fluorescent stains to separately identify platelets and leukocytes adds information useful to study the cell adhesion mechanisms associated with cross-species coagulation pathway dysregulation (physiologically inappropriate thrombosis, platelet activation and adhesion) and integrin- and selectin-mediated WBC activation and adhesive interactions. This technique can be applied to efficiently evaluate potentially important interactions between these cell populations that may contribute to graft injury mechanisms using a small sample of donor endothelial cells and a few milliliters of recipient blood.
To cite this abstract in AMA style:
Connolly MR, Petitpas K, Burdorf L, Cerel B, Laird C, Harris D, Azimzadeh A, III RNPierson. Novel Technique to Analyze Blood-Endothelial Cell Interactions Under Flow Conditions [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/novel-technique-to-analyze-blood-endothelial-cell-interactions-under-flow-conditions/. Accessed February 24, 2026.« Back to 2020 American Transplant Congress