Cyclophilin-D Inhibition Mitigates Cardiac Allograft Ischemia-Reperfusion Injury and Rejection
1Pathology & Laboratory Medicine, University of Western Ontario, London, ON, Canada, 2London Health Sciences Center, London, ON, Canada
Meeting: 2020 American Transplant Congress
Abstract number: B-353
Keywords: Apoptosis, Endothelial cells, Graft survival, Necrosis
Session Information
Session Name: Poster Session B: Ischemia Reperfusion & Organ Rehabilitation
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: The clinical relevance of ischemia-reperfusion injury (IRI) in the context of organ transplantation is well-established. IRI is associated with various forms of programmed cell death (PCD), of which necroptosis is particularly significant. Necroptosis is an inflammatory form of PCD that promotes alloimmunity and adversely affects allograft viability and function. We have recently shown the role of Cyclophilin D (CypD), a critical mediator of mitochondrial permeability transition pore (mPTP) formation, in microvascular endothelial cell (MVEC) necroptosis. In this study, we investigated the downstream mechanism of CypD-mediated necroptosis. Furthermore, we studied the role of CypD inhibition in mitigating IRI-induced allograft injury and the subsequent alloimmune response in a clinically relevant scenario of cardiac transplantation.
*Methods: To model ischemic insult in vitro, MVECs were exposed to hypoxia in glucose-free medium. To model the reperfusion event, MVECs were then transferred to a normoxic incubator in cell culture medium. Cell death was monitored by live-cell imaging and flow cytometry. For in vivo studies, C57BL/6 heart grafts were subjected to cold ischemia storage before transplantation into BALB/c mice. Histopathological grading of IRI and allograft rejection was done by a pathologist.
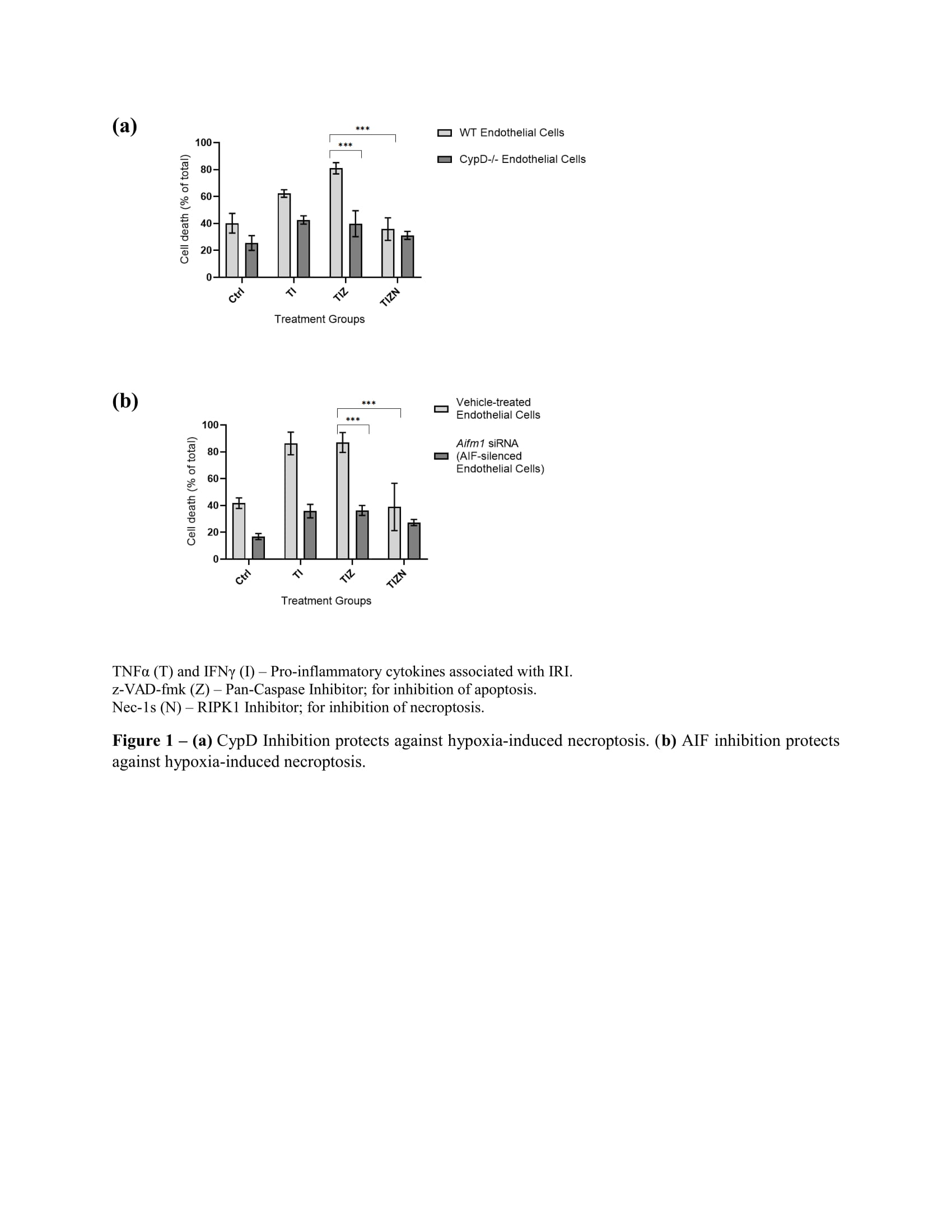
*Results: Our data indicate that inhibition of caspases during hypoxia decreases apoptosis but increases necroptosis and that inhibition of CypD attenuates hypoxia-induced necroptosis (n=3; p≤0.001). Interestingly, we found that hypoxia-induced necroptosis involves apoptosis-inducing factor (AIF) translocation to the nucleus and that AIF silencing also attenuates hypoxia-induced necroptosis (n=3; p≤0.001). Our in vivo studies confirm that CypD deficiency in ischemia-treated donor hearts mitigates IRI and allograft rejection (n=8; p=0.008).
*Conclusions: Our findings suggest that CypD inhibition following transplantation substantially attenuates necroptosis and mitigates allograft injury and the subsequent alloimmune response. Our data also indicate that AIF may be the downstream effector molecule that executes IRI-induced DNA damage in necroptosis. As such, targeting mitochondrial permeability may be a plausible approach in formulating therapeutic strategies aimed at improving allograft viability and function.
To cite this abstract in AMA style:
Qamar A, McLeod P, Jiang J, Huang X, Jevnikar AM, Zhang Z. Cyclophilin-D Inhibition Mitigates Cardiac Allograft Ischemia-Reperfusion Injury and Rejection [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/cyclophilin-d-inhibition-mitigates-cardiac-allograft-ischemia-reperfusion-injury-and-rejection/. Accessed February 24, 2026.« Back to 2020 American Transplant Congress