Improved Platelet Sequestration with Humanization of Von Willebrand Factor in Pig Xenogeneic Lung Perfusion Model
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, 2University of Maryland, Baltimore, Baltimore, MD, 3Revivicor, Blacksburg, VA
Meeting: 2020 American Transplant Congress
Abstract number: A-274
Keywords: Lung, Lung transplantation, Protective genes, Rejection
Session Information
Session Name: Poster Session A: Xenotransplantation
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Von Willebrand factor (vWF) is an important element of hemostasis and when activated, mediates platelet adhesion and promotes platelet aggregation under shear stress conditions. While human vWF requires an activation step, porcine vWF (pvWF) behaves as if “always active” when exposed to human blood, leading to non-physiologic platelet adhesion and activation. We hypothesized that pig lungs expressing humanized vWF (hvWF) would have less coagulation cascade activation and platelet sequestration when perfused with human blood.
*Methods: GalTKO.hCD46.hvWF transgenic lungs, in which multiple specific GP1b-binding domains of the porcine vWF were replaced with the human ortholog sequences, were perfused in an ex vivo model with freshly collected human blood and compared to reference GalTKO.hCD46 lungs, lacking the vWF modification. In pairs of lungs from each pig, blood was left ‘untreated’ (n=5 hvWF, n=5 reference) on one side, receiving our standard 1-BIA (thromboxane synthase inhibitor), anti-GPIb Fab, and histamine receptor blockade, or it was ‘treated’ with the addition of an integrin (H52/IB4) and selectin (GMI-1271, PSGL-1) blocking cocktail (n=5 hvWF, n=5 reference). Experiments were terminated electively after 8 hours of perfusion if lungs had not failed earlier. Platelet counts were measured by flow cytometry.
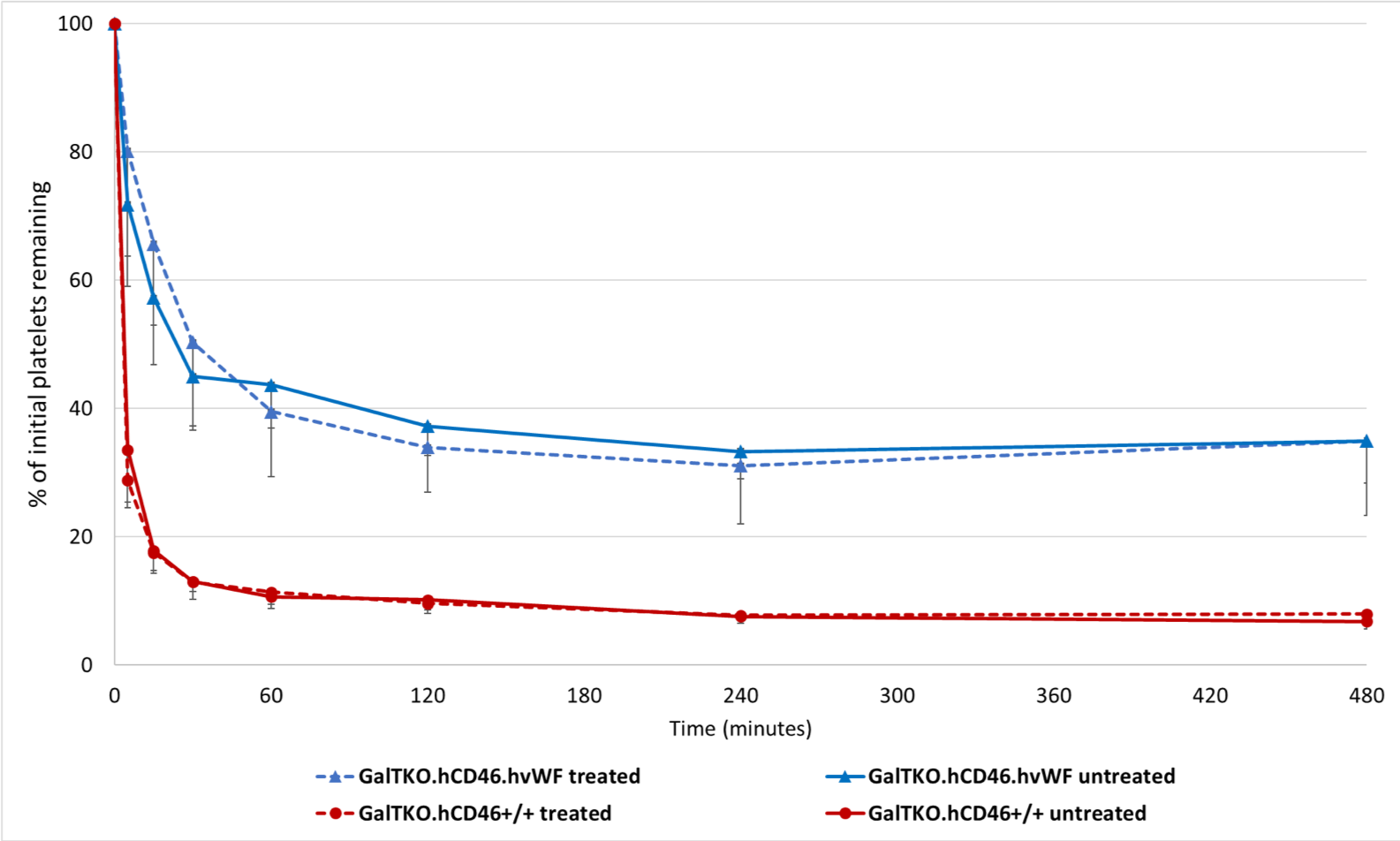
*Results: All groups showed similar survival time with most experiments reaching the time of elective termination. Pulmonary vascular resistance remained very low and constant in both cohorts. Platelet sequestration was significantly reduced and delayed in lungs with hvWF compared to those with pvWF, which was not modulated by selectin and integrin blocking treatment (p<0.01) (Fig.1). No significant differences were observed in BTG (platelet activation marker) levels, F1+2 (thrombosis marker) levels, and neutrophil counts.
*Conclusions: Incompatibility of pvWF and human platelets plays a major role in platelet sequestration, which is attenuated but not entirely prevented by the human vWF ortholog. Current work to identify selectin- and integrin-independent platelet adhesion mechanisms will focus on Fc-receptors, adenosine pathway dysregulation, activated neutrophils, and coagulation pathway dysregulation, through additional drug treatments and genetic modifications.
To cite this abstract in AMA style:
Connolly MR, Burdorf L, Abady Z, Sendil S, Redding E, Cerel B, Pratts S, Phelps C, Eyestone W, Ayares D, Azimzadeh A, III RNPierson. Improved Platelet Sequestration with Humanization of Von Willebrand Factor in Pig Xenogeneic Lung Perfusion Model [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/improved-platelet-sequestration-with-humanization-of-von-willebrand-factor-in-pig-xenogeneic-lung-perfusion-model/. Accessed January 14, 2026.« Back to 2020 American Transplant Congress