Carfilzomib-Based Desensitization: Effects on HLA, Non-HLA, Auto, and Antiviral Antibodies
1U Cincinnati, Cincinnati, OH, 2U Cincinnati, Christ Hospital, Cincinnati, OH
Meeting: 2020 American Transplant Congress
Abstract number: 571
Keywords: Antibodies, Antilymphocyte antibodies, Endothelial cells, Immunoglobulins (Ig)
Session Information
Session Name: Kidney Immunosuppression: Desensitization
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:51pm-4:03pm
Presentation Time: 3:51pm-4:03pm
Location: Virtual
*Purpose: An important question in plasma cell (PC) therapies is the specificity of therapy for PC populations. It is known that plasmablast populations are exquisitely sensitive to proteasome inhibitor (PI) therapy, whereas long-lived PC populations are resistant. Furthermore, for long-standing humoral responses, it is not clear whether HLA, non-HLA (nHLA), auto, antibodies (AAb) and protective antibodies (Ab) are affected differently by PI therapy. Therefore, we conducted an analysis of patients undergoing desensitization for nHLA, AAb, and protective Abs.
*Methods: Highly sensitized renal transplant candidates enrolled in a carfilzomib-based desensitization trial had their serum tested for nHLA (AT1R, ETAR, MICA OneLambda), AAb (LabScreen Autoantibody, One Lambda), and measles, mumps, rubella, and varicella (MMRV) antibodies (Zeus Scientific). All patients received 2 weekly doses of carfilzomib, escalating from 20mg/m2 to 36mg/m2 over 12 weeks, with 6 patients receiving weekly pheresis (group B), and 2 patients receiving weekly pheresis and 1 dose of rituximab (group C). Patients who were positive at baseline had their antibody levels assessed until day 141.
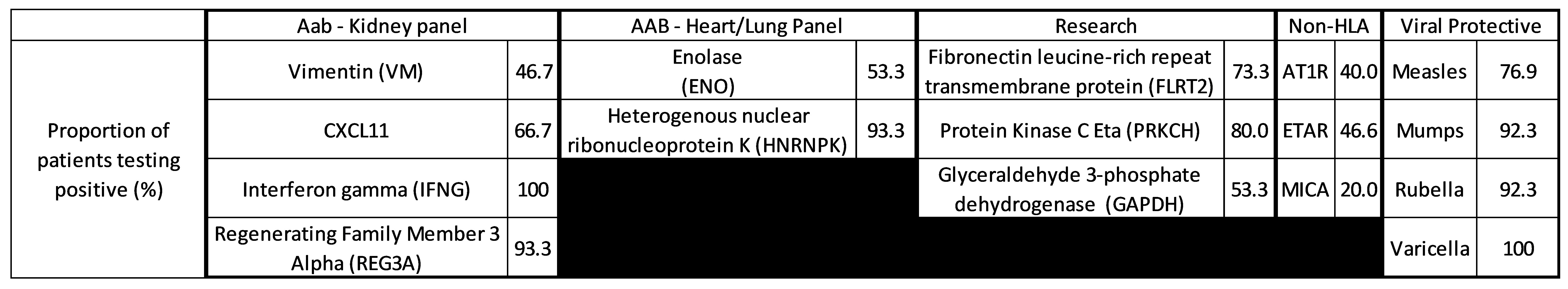
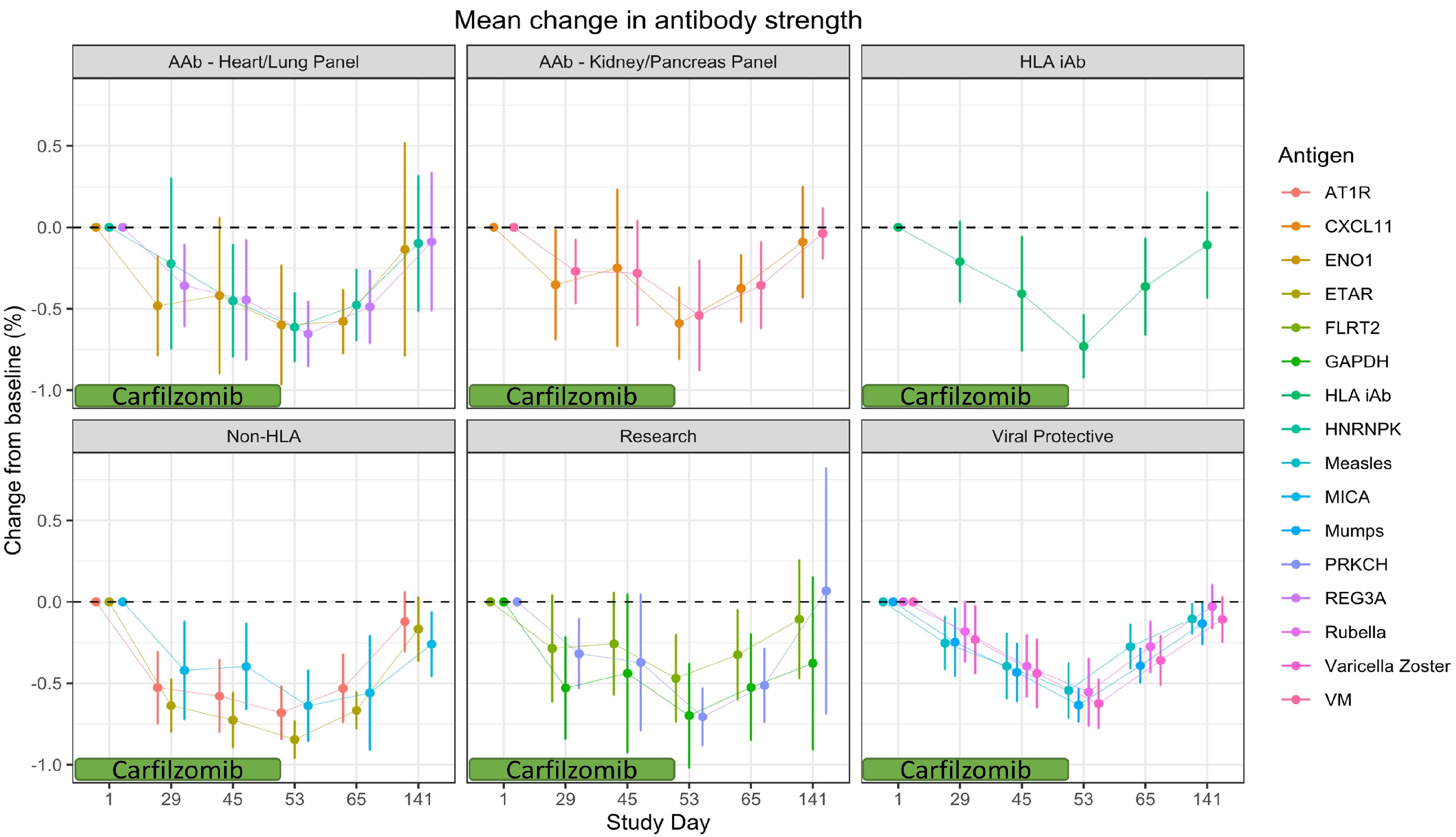
*Results: 15 patients were tested for nHLA and AAb, and 13 for MMRV antibodies. All patients tested positive for at least one antibody (median 16, range 8-28 specificities). Table 1 presents some of the most commonly-identified antibodies at baseline. By day 53, all antibodies were reduced by therapy (median 62.5%, range 47.0-84.6%). Rebound was apparent for all specificities, including protective antibodies, by day 141. Figure 1 shows the antibody level trajectory per antibody group across time.
*Conclusions: These data indicate that for long standing humoral responses, PI therapy does not appear to differentially affect Ab levels based on the general type of Ab. HLA, nHLA, AAb, and protective antibodies responded similarly to carfilzomib therapy, with a rebound similar to what has been observed with HLA Abs. In addition, protective immunity returned to baseline following cessation of PI therapy.
To cite this abstract in AMA style:
Tremblay S, Portwood E, Shields AR, Alloway RR, Brailey PA, Woodle E. Carfilzomib-Based Desensitization: Effects on HLA, Non-HLA, Auto, and Antiviral Antibodies [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/carfilzomib-based-desensitization-effects-on-hla-non-hla-auto-and-antiviral-antibodies/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress