Preclinical Study for Liver Allograft Tolerance after Rapid Immunosuppression Withdrawal Using Cynomolgus Monkeys
1Columbia Center for Translational Immunology, Department of Medicine, Columbia University, New York, NY, 2Department of Pediatrics, Columbia University, New York, NY, 3Departments of Pathology and Cell Biology, Columbia University, New York, NY, 4Department of Surgery, Columbia University, New York, NY
Meeting: 2020 American Transplant Congress
Abstract number: 468
Keywords: Liver transplantation, Preclinical trails, Tolerance
Session Information
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:15pm-4:27pm
Presentation Time: 4:15pm-4:27pm
Location: Virtual
*Purpose: Operational tolerance of liver allografts occurs in highly selected patients but cannot be reliably induced. Herein, we investigated if tolerance to liver can be achieved in cynomolgus macaques after rapid immunosuppression (IS) withdrawal with non-myeloablative preparative regimens.
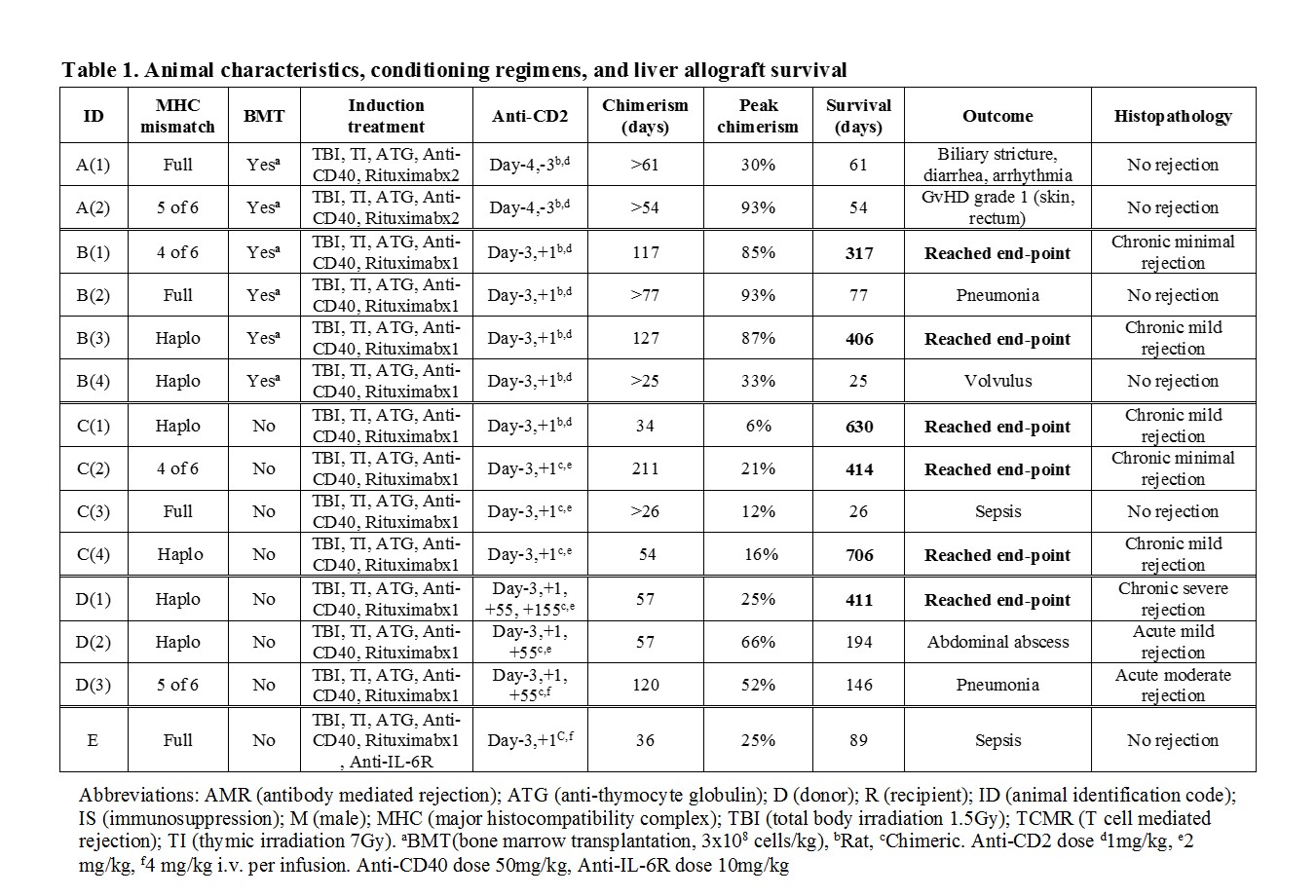
*Methods: 14 cynomolgus monkeys received liver transplants with or without donor bone marrow transplantation (BMT) (Table 1). Subjects received tacrolimus-based IS maintenance for 28 days and then complete withdrawal on day 55. We systematically evaluated peripheral blood chimerism, graft function and immunological status until an end-point was reached. Endpoints were graft rejection, graft-versus-host disease (GVHD), weight loss, survival >600 days or death. Protocols were modified based on outcomes.
*Results: Multilineage mixed chimerism was seen in recipients with or without BMT. Animal A(2) developed grade 1 GVHD. The addition of anti-CD2 mAb on POD1 in Group B depleted donor CD8+ T effector memory (Tmem) and prevented GVHD while permitting mixed chimerism and extended IS-free graft survival. In Group C, without BMT, mild rejection was seen long-term, even when chimerism was lost and Tmem cells recovered. Additional doses of anti-CD2 mAb on POD55 in Group D did not deplete recovered Tmem due to elicited antibodies against anti-CD2 mAb. Increased serum IL-6 levels during a period of bacterial infection correlated with the expansion of Tmem associated with a rejection crisis, while the expansion of Tmem was absent in Animal E that received anti-IL-6R mAb. PD-L1 expression on antigen-presenting cells in the liver allograft was upregulated in recipients with long-term survival.
*Conclusions: This is the first proof of concept study demonstrating long-term stable liver allograft function in nonhuman primates using a non-myeloablative treatment regimen to facilitate rapid IS withdrawal. The liver’s ability to modulate the activation of host T cells by PD-L1/PD-1 pathway may contribute to tolerance of liver allografts.
To cite this abstract in AMA style:
Sakai H, Berglund E, Chaudhry S, Kato Y, Weiner J, Alonso-Guallart P, Llore N, Bruestle K, Fredriksson F, Stern J, Ekanayake-Alper D, Martinez M, Pierre G, Danton M, Kofman S, Ordanes D, Lefkowitch J, Iuga A, Kato T, Sykes M, Griesemer A. Preclinical Study for Liver Allograft Tolerance after Rapid Immunosuppression Withdrawal Using Cynomolgus Monkeys [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/preclinical-study-for-liver-allograft-tolerance-after-rapid-immunosuppression-withdrawal-using-cynomolgus-monkeys/. Accessed February 26, 2026.« Back to 2020 American Transplant Congress