Efficacy Of Rituximab Monotherapy On Anti-human Leukocyte Antigen Antibodies In Highly Sensitized Living Donor Kidney Transplant Patients.
1Clinical Pharmacy, King Faisal Specialist Hospital and Research Center (KFSH&RC), Riyadh, Saudi Arabia, 2Clinical Pharmacy, KFSH&RC, Riyadh, Saudi Arabia, 3Kidney and Pancreas transplantation, KFSH&RC, Riyadh, Saudi Arabia, 4kFSH&RC, Riyadh, Saudi Arabia, 5Clinical Director, Histocompatibility and Immunogenetics Laboratory, KFSH&RC, Riyadh, Saudi Arabia, 6KFSH&RC, Riyadh, Saudi Arabia, 7Riyadh Elm University, Riyadh, Saudi Arabia, 8Clinical Transplant Coordinator, KFSH&RC, Riyadh, Saudi Arabia
Meeting: 2019 American Transplant Congress
Abstract number: B185
Keywords: B cells, CD20, HLA antibodies, Kidney transplantation
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Desensitization
Session Type: Poster Session
Date: Sunday, June 2, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: We aim to examine the efficacy of Rituximab (RTX) monotherapy in reducing DSA in Highly Sensitised Living Donor Kidney Transplant (HS-LDKT) candidates and potentially improve their chance of receiving a suitable organ.
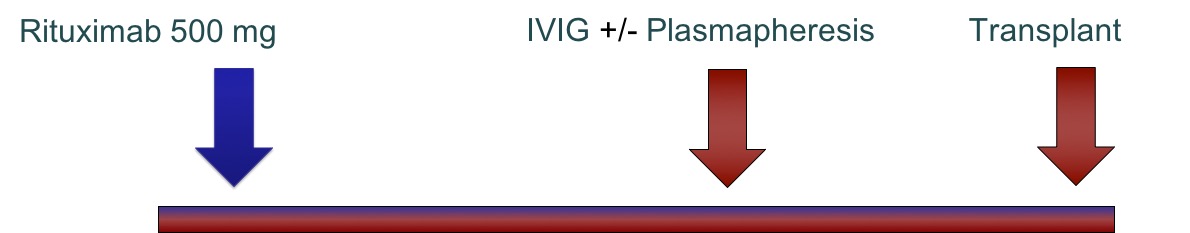
*Methods: Study Design: a retrospective cohort study of all sensitized patients who received RTX therapy before kidney transplantation and met the inclusion criteria (HS adults and received RTX monotherapy). Patients were excluded if they were pediatrics, underwent other organ transplantation, received RTX/IVIG at the same time, were pregnant or on the deceased donor list. The study protocol was approved by the institutional review board (IRB). Our desensitization protocol includes RTX 500 mg IV infused over 10 hours then IVIG 2 g/kg before transplant surgery and +/- plasmapheresis sessions. Study duration was from 01-Jan-2008 through 31-Dec-2016. The primary endpoint was the percentage reduction in MFI-DSA in HS-LDKT patientsafter receiving one dose RTX. Secondary endpoints included patients and graft survival, acute antibody mediated rejection (AMR) and adverse reactions. Patients’ sera were analyzed using the highly sensitive microbeads-based Luminex assay to compare both class I and class II HLA DSA, which was assessed using Mean Fluorescence Intensity (MFI), before and after RTX, but prior to IVIG administration. Subjects were followed using an intention to treat analysis. We included the whole population of patients who met the selection criteria and hence, sample size calculation was not performed in a priori fashion. However, power analysis was calculated in a post-hoc manner.A pvalue of < 0.05 was used to define statistical significance.
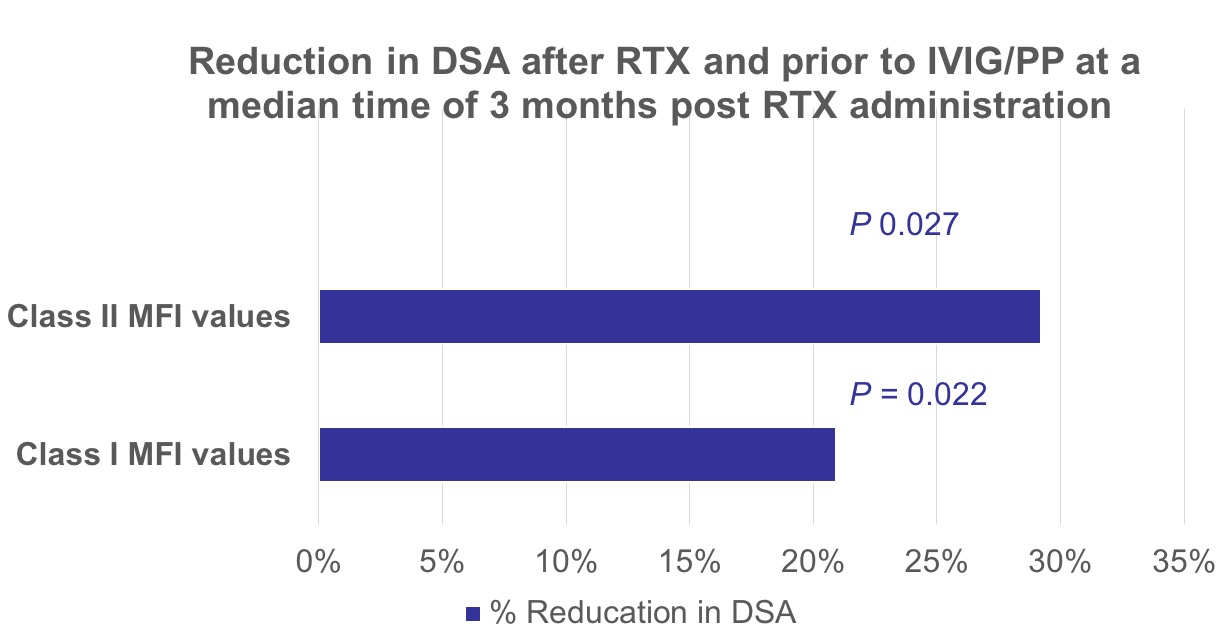
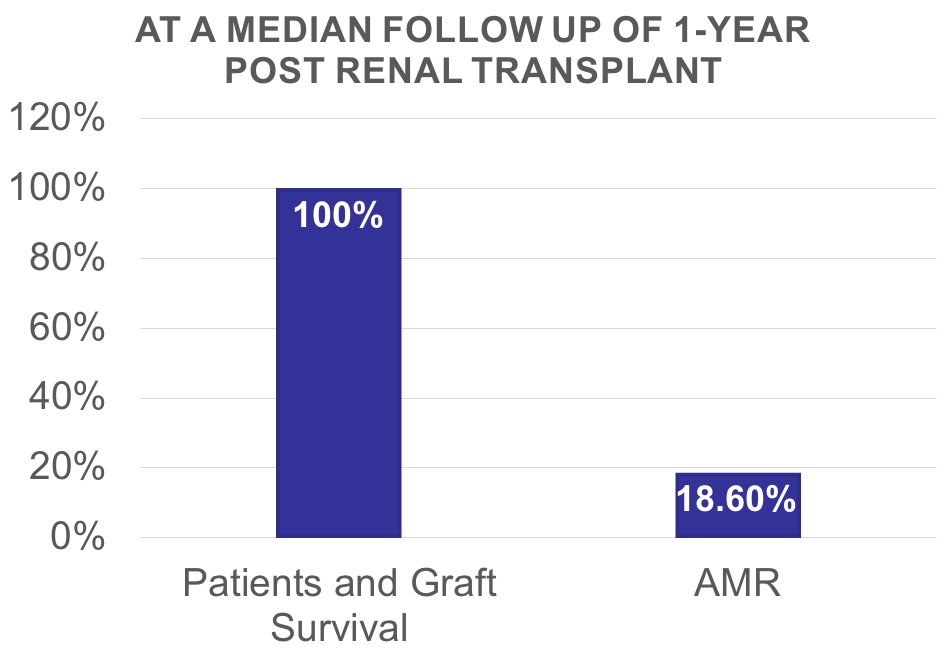
*Results: 103 HS patients who underwent desensitization with RTX and IVIG were identified. Of these, 70 patients (68%) met the inclusion criteria and were included in the intention to treat analysis. Fifty-seven patients (81%) had anti-HLA antibodies against class 1, while 41 patients (58.5%) had anti-HLA class II. Desensitization with RTX alone decreased MFI values of class I HLA DSA by a median of 20.9% and class II HLA DSA by a median of 29.2% at a median time of 3 months post RTX administration. All patients were successfully transplanted after receiving the remainder of desensitization protocol i.e. IVIG and plasmapheresis. No major adverse events were identified. Mean ± SD serum creatinine was 79.5 ± 16.7 µmol/L at 1 year.
*Conclusions: This study suggests that desensitization using RTX administration in LDKThelps cross the HLA-barrier but it should be implemented as part of a holistic desensitization approach. Larger and randomized clinical trials addressing antibody reduction with B-cell depletion are required to support this study’s findings.
To cite this abstract in AMA style:
Sindi DJ, Jedai AAl, Aleid H, Brockmann J, Al-Awwami M, Alissa D, Vol EDe, Khabbaz HAl, Fajji L. Efficacy Of Rituximab Monotherapy On Anti-human Leukocyte Antigen Antibodies In Highly Sensitized Living Donor Kidney Transplant Patients. [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/efficacy-of-rituximab-monotherapy-on-anti-human-leukocyte-antigen-antibodies-in-highly-sensitized-living-donor-kidney-transplant-patients/. Accessed February 9, 2026.« Back to 2019 American Transplant Congress