Donation After Circulatory Death Kidneys With Prolonged Delayed Graft Function Present Widespread Metabolic And Translational Deficiencies At Time Of Retrieval
1Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom, 2University Medical Centre Groningen, Groningen, Netherlands, 3Oxford Transplant Centre, Oxford, United Kingdom
Meeting: 2019 American Transplant Congress
Abstract number: 601
Keywords: Kidney, Renal injury, Renal ischemia
Session Information
Session Name: Concurrent Session: Ischemia Reperfusion & Organ Rehabilition III
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:54pm-5:06pm
Presentation Time: 4:54pm-5:06pm
Location: Room 313
*Purpose: Delayed graft function (DGF) is acute renal failure post-transplant, commonly defined as the need for dialysis in the first week post-transplant. Injury at time of death affects graft quality in different ways. Donor, recipient, preservation factors and IRI all contribute to DGF, but there is currently no way of predicting DGF or its duration. This study investigated the biological pathways in DCD kidneys at time of donation that related to DGF and could discriminate different DGF durations.
*Methods: N=30 DCD kidney biopsies were selected from the UK Quality in Organ Donation (QUOD) biobank and stratified according to outcome and DGF duration (immediate function, IF n=10, short DGF (1-6 days), SDGF n=10; long DGF (7-22 days), LDGF n=10). Samples were matched for donor and recipient age, gender, BMI (<30), f-WIT, no donor AKI and CIT (≤ 18h). Proteins were extracted and analysed by LC-MS/MS proteomics. Pathway analysis was run by Ingenuity Pathway Analysis. Correlations between protein levels and DGF duration were studied by Pearson correlation.
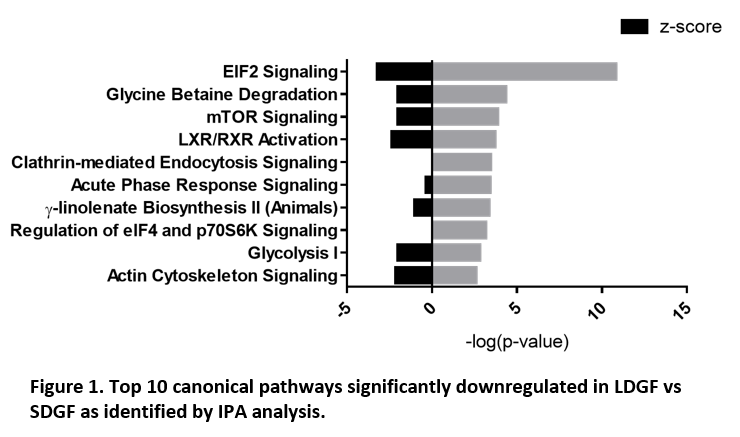
*Results: 3,999 proteins were identified and n=418, n=181 and n=374 were significantly different (p<0.05, unpaired t-test) in SDGF vs IF, LDGF vs IF and LDGF vs SDGF respectively. SDGF kidneys presented activation of stress pathways geared towards cell survival (eIF2 and autophagy signalling) when compared to IF, while LDGF kidneys presented impaired response to stress (downregulation of Nrf2-mediated oxidative stress response). eIF2, mTOR signalling and glycolysis were all downregulated in LDGF vs SDGF (Figure 1). Histone H3.3, which accumulates at sites of DNA injury, was increased in LDGF and its levels correlated with DGF duration (Pearson r 0.7224).
*Conclusions: DCD kidneys with short duration of DGF present acute cellular injury at time of donation, alongside upregulation of repair pathways (e.g. chaperone-mediated autophagy, eIF2-dependent protein translation). In contrast, DCD kidneys with prolonged DGF present widespread translational, metabolic and antioxidant deficiencies. These pathways could be targeted therapeutically to reduce DGF incidence and duration.
To cite this abstract in AMA style:
Faro MLLo, Rozenberg K, Huang H, Maslau S, Leuvenink H, Sharples E, Ploeg R. Donation After Circulatory Death Kidneys With Prolonged Delayed Graft Function Present Widespread Metabolic And Translational Deficiencies At Time Of Retrieval [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/donation-after-circulatory-death-kidneys-with-prolonged-delayed-graft-function-present-widespread-metabolic-and-translational-deficiencies-at-time-of-retrieval/. Accessed February 15, 2026.« Back to 2019 American Transplant Congress