MDSCs Prolong Allogeneic Cardiac Graft Survival by Regulating Interferon Type 1 Signaling
1Brigham and Women's Hospital, Harvard Medical School, Boston, MA, 2Boston Children's Hospital, Harvard Medical School, Boston, MA
Meeting: 2019 American Transplant Congress
Abstract number: 598
Keywords: Allorecognition, Heart/lung transplantation, Immunosuppression, T cell reactivity
Session Information
Session Name: Concurrent Session: Stem Cell, Cellular Therapies and Regenerative Medicine
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: Room 310
*Purpose: We previously demonstrated that donor-derived regulatory dendritic cells (DCreg), contrary to recipient derived DCreg, promote antigen-specific allograft protection. As myeloid-derived suppressor cells (MDSC) show an enhanced immune suppressive capability, we hypothesize that donor-type MDSC suppress alloimmunity by regulating donor-specific T cell clones.
*Methods: MDSC were generated from BALB/c bone marrow cells. Allogeneic mix lymphocyte reaction (alloMLR) and regulatory T cells (Treg) induction assays were performed in vitro. B6 recipients were pre-treated with BALB/c MDSCs or conventional DCs (cDC) control. Subsequently, these mice were transplanted with either donor-type (BALB/c) or third-party (C3H) cardiac grafts. We then examined graft survival, allo-rejection/tolerance associated immunophenotype and graft mRNA expression.
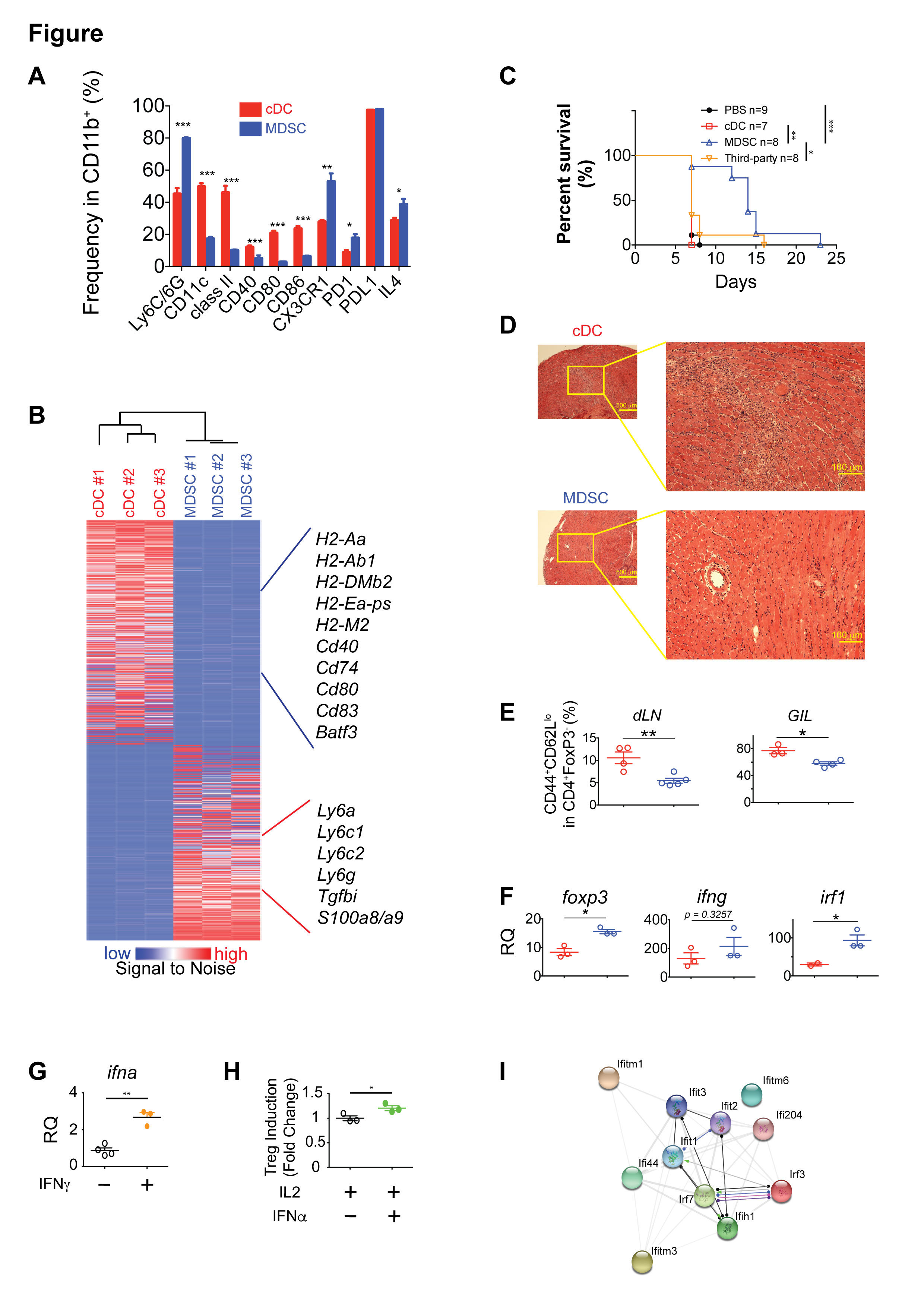
*Results: Flowcytometry showed increased level of CX3CR3, PD1, IL4 and decreased level CD40/80/86, MHC class-II in MDSC when compared to cDC (fig-A). RNA-seq showed an upregulation of Tgfbi and s100A8/A9 (fig-B). MDSC significantly suppressed the proliferation of alloreactive CD4+ and CD8+ T cells in alloMLR.
MDSC treated group (MG) had a prolonged BALB/c cardiac graft survival when compared to other groups (fig-C). HE staining revealed a significant decrease of allograft infiltrating lymphocytes (GIL) in MG (fig-D). The proportion of CD4+ effector memory T cells (TEM) within GIL and draining lymph nodes (dLN) was significantly decreased in MG (fig-E). The mRNA expression analysis revealed an interesting observation of FoxP3 upregulation along with IFNg and IRF1 in MG’s allografts (fig-F).
To better understand the mechanisms involved, we simulated allorejection by stimulating MDSC with IFNg in vitro and performed RNA-seq. When we analyzed upregulated genes by using STRING database, we found an enrichment of type I IFN related genes that showed an active interaction pattern (fig-I). Interestingly, stimulating MDSC with IFNg resulted in upregulation of secretion of IFNa by MDSC (fig-G). Moreover, we found that IFNa preferentially induce Treg and suppress the proliferation of non-Treg both in vitro (fig-H) and in vivo.
*Conclusions: MDSC protect cardiac allograft from acute rejection by suppressing TEM, and inducing Treg in a donor antigen specific manner. MDSC induce immune regulation by transforming inflammatory IFNg into regulatory IFNa signaling that preferentially promote Treg.
To cite this abstract in AMA style:
Cai S, Sage PT, Borges TJ, Ichimura T, Eskandari SK, Choi JY, Salkuj I, Dulaijan BSAI, Allos H, Miao J, Riella LV, Chandraker AK, Azzi JR. MDSCs Prolong Allogeneic Cardiac Graft Survival by Regulating Interferon Type 1 Signaling [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/mdscs-prolong-allogeneic-cardiac-graft-survival-by-regulating-interferon-type-1-signaling/. Accessed February 18, 2026.« Back to 2019 American Transplant Congress