Using a Commercially Available Assay That Measures Cytomegalovirus-Specific CD4+ and CD8+ T-cell Immunity to Predict Clinically Significant Cytomegalovirus Events in Solid Organ Transplant Recipients
1Infectious Diseases, Brown University, Providence, RI, 2Infectious Diseases, University of Maryland, Baltimore, MD, 3Internal Medicine, Brown University, Providence, RI
Meeting: 2019 American Transplant Congress
Abstract number: 335
Keywords: Cytomeglovirus, Multicenter studies, T cell reactivity
Session Information
Session Name: Concurrent Session: Breakthroughs in Cytomegalovirus
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:30pm-5:42pm
Presentation Time: 5:30pm-5:42pm
Location: Ballroom A
*Purpose: To assess the diagnostic performance and clinical utility of the Viracor CMV T-cell Immunity Panel (CMV-TCIP), which measures both CMV-specific Cd4+ and Cd8+ T-cell responses using flow cytometry and intracellular cytokine staining, to predict clinically significant CMV events in solid organ transplant (SOT) recipients.
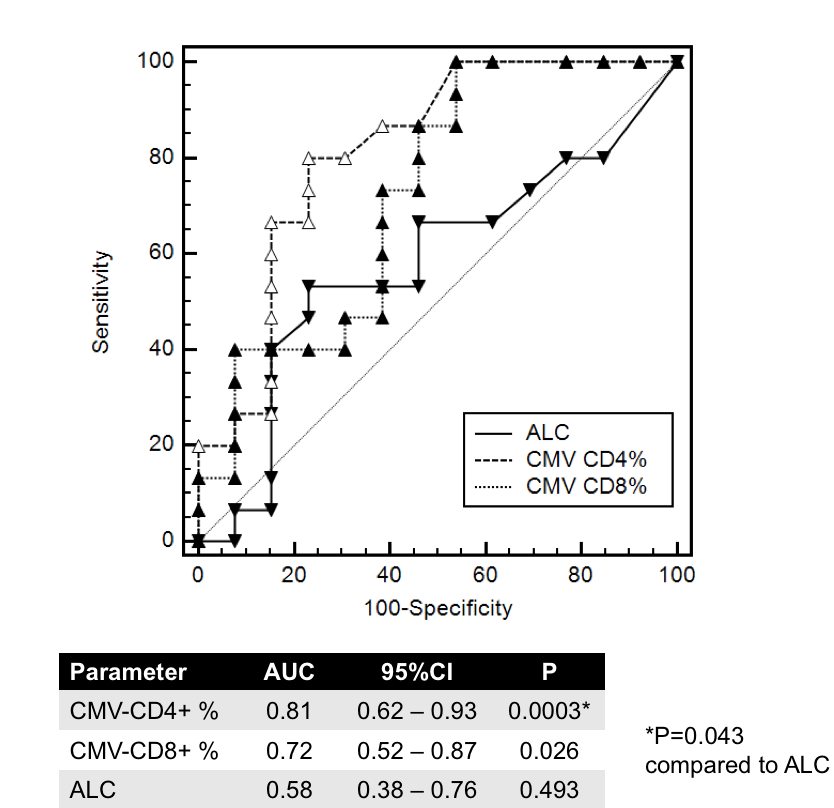
*Methods: We enrolled SOT recipients receiving care at either Brown University or University of Maryland affiliated hospitals between 04/2017 and 10/2018 who had CMV-TCIP results and at least one subsequent assessment for CMV DNAemia. A CMV event was defined as CMV DNAemia prompting initiation of treatment. We built CMV-protection relative operating curves (ROC) for CMV-specific %Cd4+ and %Cd8+ T-cells as well as for absolute lymphocyte count (ALC).
*Results: We analyzed 28 samples from 21 SOT recipients [20(16) kidney, 6(3) heart, 2 lung; 10(8) CMV R+, 18(13) D+/R-]. The CMV-protection ROC-AUC was significant for %CMV-specific Cd4+ and Cd8+ T-cells, but not for ALC (Figure). At a cut-off of 0.22% CMV-specific Cd4+ T-cells, PPV was 80% (95% CI 51-96%) and NPV was 77% (95% CI 45-95%). The 3 patients with “false positive” %CMV-specific Cd4+ T-cells were classified as such because of valganciclovir initiation, but none had symptoms due to CMV; in 2 of these patients, CMV DNAemia was decreasing before treatment.
*Conclusions: In this small observational study the CMV-TCIP, but not the ALC, was useful in predicting subsequent CMV events deemed significant by the treating physicians. The clinical utility of this commercially available assay merits further validation in a blinded prospective study.
To cite this abstract in AMA style:
Rogers R, Saharia K, Chandorkar A, Weiss Z, Farmakiotis D. Using a Commercially Available Assay That Measures Cytomegalovirus-Specific CD4+ and CD8+ T-cell Immunity to Predict Clinically Significant Cytomegalovirus Events in Solid Organ Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/using-a-commercially-available-assay-that-measures-cytomegalovirus-specific-cd4-and-cd8-t-cell-immunity-to-predict-clinically-significant-cytomegalovirus-events-in-solid-organ-transplant-recipients/. Accessed February 9, 2026.« Back to 2019 American Transplant Congress