Deceased Donor and Class II Donor Specific Antibody Predict Interface Activity While Increased Age at Time of Biopsy Predicts Fibrosis in Long-Term Pediatric Liver Allografts With Normal Liver Tests: iWITH Clinical Trial
1UCSF, SF
2U Cincinnati, Cincinnati
3U Pittsburgh, Pittsburgh
4Rho, Chapel Hill
5ITN, Bethesda
6NIAID/NIDDK/ITN, Bethesda.
Meeting: 2015 American Transplant Congress
Abstract number: 156
Keywords: Alloantibodies, Fibrosis, Histology, Inflammation
Session Information
Session Time: 8:30am-9:30am
 Presentation Time: 9:15am-9:30am
Presentation Time: 9:15am-9:30am
Location: Terrace Ballroom 1, 2, 3
Background: Although multiple single centers have reported deterioration of pediatric liver grafts in the face of normal liver tests, neither risk nor mechanism is understood.
Methods: iWITH is a prospective, 12 center trial of immunosuppression (IS) withdrawal trial for pediatric liver transplant (tx) recipients with ALT and GGT <50IU/L. LabScreen Single Antigen assays defined antibody specificities. A central pathologist blindly scored eligibility biopsies (bxs) for 48 histologic criteria and C4d staining intensity (0-3 each for portal capillary and vein, sinusoidal, and central vein). Three dominant features informed hierarchical cluster analysis: interface activity (IA), peri-portal, and peri-venular fibrosis. Using clinical covariates, C4d scores, and donor specific antibody (DSA) presence, multivariable logistic regression analysis and backward variable selection (p<0.10 for retention) identified independent predictors of cluster assignment.
Results: 157 recipients (79M/78F) of 47 living/110 deceased (74 whole/36 partial) donor txs at 1.8±1.7ys underwent bx at 10.7±3.5ys; ALT and GGT were 28±17 and 19±20IU/L. Bxs segregated into 3 distinct clusters. I) IA±fibrosis: n=34; II) Fibrosis/No IA: n=44; III) No IA/No fibrosis: n=79 (Fig). Among donor (age, sex, living/deceased, & whole/partial graft) and recipient (age at tx & bx, sex, liver disease, induction & current IS, rejection history, ALT, GGT, & DSA) factors, only deceased donor and Class II DSA predicted assignment to Cluster I; only recipient age at bx predicted assignment to Cluster II (Table).
Conclusions: Stable long-term pediatric liver grafts with normal liver tests can harbor significant histopathological changes. Identification of discrete histologic clusters with unique clinical correlates suggests distinct immunologic and non-immunologic mechanisms of allograft deterioration.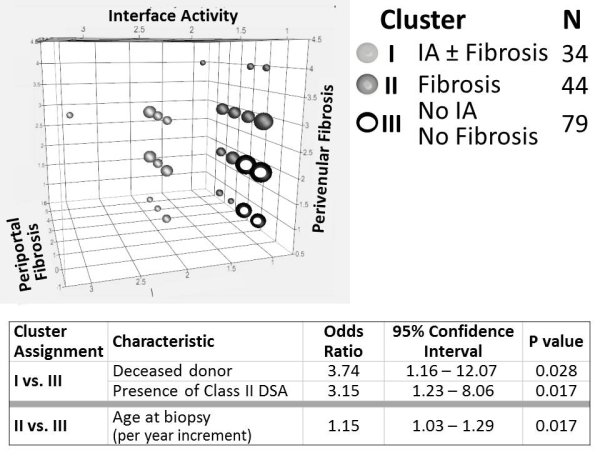
To cite this abstract in AMA style:
Feng S, Bucuvalas J, Demetris A, Spain K, Mazariegos G, Burrell B, Investigators iWITH. Deceased Donor and Class II Donor Specific Antibody Predict Interface Activity While Increased Age at Time of Biopsy Predicts Fibrosis in Long-Term Pediatric Liver Allografts With Normal Liver Tests: iWITH Clinical Trial [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/deceased-donor-and-class-ii-donor-specific-antibody-predict-interface-activity-while-increased-age-at-time-of-biopsy-predicts-fibrosis-in-long-term-pediatric-liver-allografts-with-normal-liver-tests/. Accessed February 16, 2026.« Back to 2015 American Transplant Congress
