Curative Correction of FAH Deficiency by Chimeric Stem Cells Transplantation
1Division of Transplant Surgery, Mayo Clinic, Rochester, MN
2Department of Surgery, Yonsei University College of Medicine, Seoul, Korea
3Department of Pathology, University of Michigan, Ann Arbor, MI
4Recombinetics Inc, Saint Paul, MN
5International Center for Biotechnology, Cooperative Resources International, Mount Horeb, WI.
Meeting: 2018 American Transplant Congress
Abstract number: 507
Keywords: Liver, Pig, Stem cells
Session Information
Session Name: Concurrent Session: Cellular Therapies and to Promote Tolerance
Session Type: Concurrent Session
Date: Tuesday, June 5, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: Room 606/607
Fumarylacetoacetate hydrolase (FAH) deficient pigs have been proposed as surrogate hosts for large scale in vivo expansion of normal FAH+ hepatocytes. However, immunologic rejection prevents expansion of non-autologous donor cells. To overcome this barrier, we have developed a novel method of morula complementation to produce chimeric FAH (-/-) pigs with FAH (+/+) liver grafts.
FAH (-/-) recipient morulae were derived via somatic cell nuclear transfer with fibroblasts harvested from FAH knock-out pigs. The blastomeres of the recipient morulae were complemented with blastomeres from normal FAH (+/+) donor embryos also transfected with enhanced green fluorescent protein (EGFP) label. The chimeric embryos were transferred to a surrogate sow who received no NTBC during pregnancy.
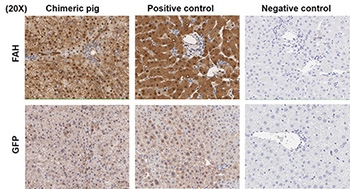
A single gestation was confirmed and the chimeric pig was born without deformity. His birth weight was 0.75 kg. Liver function tests were normal. Tyrosine and succinylacetone levels were also normal. He received no NTBC. Peripheral blood leukocytes were comprised of 33.8% donor-derived (GFP+) cells as measured via flow cytometry. Liver biopsies at 1 and 2 months after birth showed diffuse staining of donor (FAH+, GFP+) cells in liver parenchyma (Fig. 1). The pig was euthanized electively and in good health without NTBC administration for data collection at day 60 after birth.
These are proof of concept studies that FAH deficiency can be successfully corrected by stem cell transplantation in early gestation through the production of chimeric embryos. Therefore, production and robust expansion of non-autologous normal hepatocytes can be achieved in FAH deficient pigs. Our novel method avoids the need for protective drugs (i.e., NTBC), immunosuppressive drugs, or immunodeficient modifications of host pigs.
CITATION INFORMATION: Joo D., Nelson E., Zhang Y., Amiot B., Ho S., Lancto C., Carlson D., Queiroz L., Nyberg S. Curative Correction of FAH Deficiency by Chimeric Stem Cells Transplantation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Joo D, Nelson E, Zhang Y, Amiot B, Ho S, Lancto C, Carlson D, Queiroz L, Nyberg S. Curative Correction of FAH Deficiency by Chimeric Stem Cells Transplantation [abstract]. https://atcmeetingabstracts.com/abstract/curative-correction-of-fah-deficiency-by-chimeric-stem-cells-transplantation/. Accessed January 8, 2026.« Back to 2018 American Transplant Congress