Lipid Catabolism Provides Alternative Energy Source and Compensates for Mitochondrial Dysfunction in Reperfused Rat Kidneys After Ischaemia.
1Oxford Transplant Centre, NDS, University of Oxford, Oxford, United Kingdom
2Target Discovery Institute, NDM, University of Oxford, Oxford, United Kingdom
3Department of Surgery, University Medical Center Groningen, Groningen, Netherlands
4Department of Surgery and Cancer, Imperial College London, London, United Kingdom
Meeting: 2017 American Transplant Congress
Abstract number: D45
Session Information
Session Name: Poster Session D: Ischemic Injury and Organ Preservation Session III
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Introduction: To reduce morbidity and mortality on the wait list for kidney transplantation, centres are required to use older and higher risk organs including unstable DBD, ECD and DCD donors affecting function and graft survival. DCD kidneys have significantly higher rates of PNF and DGF than DBD kidneys but the underlining molecular mechanism of this acute kidney injury is not well understood.
Methods: In this study, we have induced unilateral warm ischaemia for 45min in the rat model by clamping the left renal artery kidney followed by 4h and 24h reperfusion, resp. to mimic DCD with subsequent Ischaemia Reperfusion Injury (IRI). Quantitative proteomics and metabolomics were used to measure proteins and metabolites in cortex tissue extraction at 4h and 24h after IRI.
Results: After reperfusion, tissue proteomics analyses showed molecular profiles reflecting elevated acute phase, coagulation, and complement related proteins. Fatty acid (FA) signalling was found to be a major pathway alteration following IRI in kidneys. Metabolomic analysis showed increased level of lipids and FAs, and significant changes with an altered metabolism was found on the mitochondrial level. Mitochondrial function was assessed by complex I activity, oxygen consumption and ATP levels, and appeared to be significantly impaired at 24h post IRI. Eventually, IRI caused an energy-depleted state with reduced kidney function and increased creatinine levels.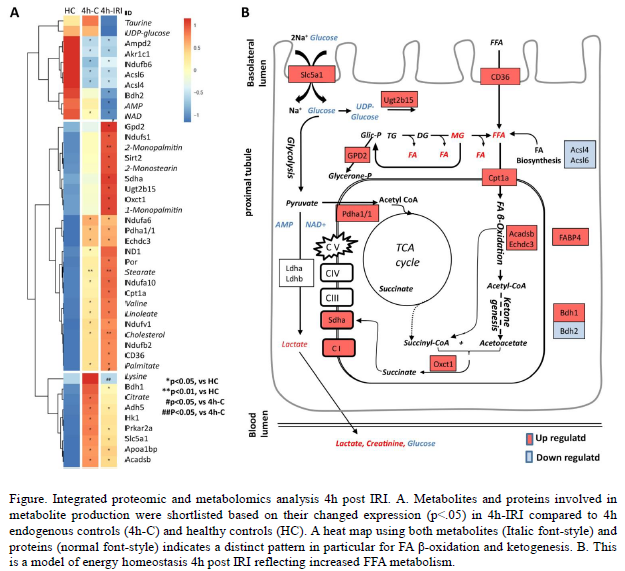 Discussion: Integrated proteo-metabolomic profiling indicates that IRI increases FA β-oxidation, which suggests a compensatory mechanism for the developing energy deficit and altered mitochondrial function. The results provide a robust framework for metabolic intervention strategies to minimise ischaemic kidney injury.
Discussion: Integrated proteo-metabolomic profiling indicates that IRI increases FA β-oxidation, which suggests a compensatory mechanism for the developing energy deficit and altered mitochondrial function. The results provide a robust framework for metabolic intervention strategies to minimise ischaemic kidney injury.
CITATION INFORMATION: Huang H, van Dullemen L, Akhtar M, Lo Faro M, Dona A, Kaisar M, Leuvenink H, Kessler B, Ploeg R. Lipid Catabolism Provides Alternative Energy Source and Compensates for Mitochondrial Dysfunction in Reperfused Rat Kidneys After Ischaemia. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Huang H, Dullemen Lvan, Akhtar M, Faro MLo, Dona A, Kaisar M, Leuvenink H, Kessler B, Ploeg R. Lipid Catabolism Provides Alternative Energy Source and Compensates for Mitochondrial Dysfunction in Reperfused Rat Kidneys After Ischaemia. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/lipid-catabolism-provides-alternative-energy-source-and-compensates-for-mitochondrial-dysfunction-in-reperfused-rat-kidneys-after-ischaemia/. Accessed March 7, 2026.« Back to 2017 American Transplant Congress
