A Pilot Trial of Genotype-Guided Dosing versus Standard Clinical Dosing in Pediatric Solid Organ Transplants.
The Hospital for Sick Children, Toronto, ON, Canada
Meeting: 2017 American Transplant Congress
Abstract number: C99
Keywords: Calcineurin, Dosage, Genomics, Immunosuppression
Session Information
Session Name: Poster Session C: Hearts and VADS: All Topics
Session Type: Poster Session
Date: Monday, May 1, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Purpose: Tacrolimus pharmacokinetics are influenced by age and variation in the CYP3A5 gene with CYP3A5 expressors (AA/AG) being fast metabolizers. The purpose of this trial was to determine if age and genotype-guided starting dose for tacrolimus in pediatric solid organ transplant (SOT) recipients results in more stable therapeutic concentrations compared to standard dosing during 30 days follow-up.
Method: 75 SOTs (heart, kidney, liver) were enrolled and underwent genotyping for CYP3A5. 53 were randomized after transplant to standard or genotype-guided starting dose in a 1:2 ratio, stratified by genotype and organ type. Frequency of out-of-target tacrolimus concentrations and time to achieve stable therapeutic concentrations was compared between the two arms using cumulative incidence function with stratification by randomization arm. Gray's test was used to assess between-stratum differences.
Results: 15 heart, 14 kidney and 24 liver transplants were randomized. 23% had CYP3A5 expressor genotype. Tacrolimus concentration at 36-48 hours post-tacrolimus initiation was 10.1±6.6 [micro]g/L in genotype arm and 13.3±8.5 [micro]g/L in standard arm. Patients in the genotype arm had fewer out-of-range concentrations during 30-day follow-up than subjects in standard arm (OR [CI] = 0.58 [0.43, 0.79], p<0.001). There was no difference between the two arms in time to achieve stable therapeutic concentrations (p=0.129). 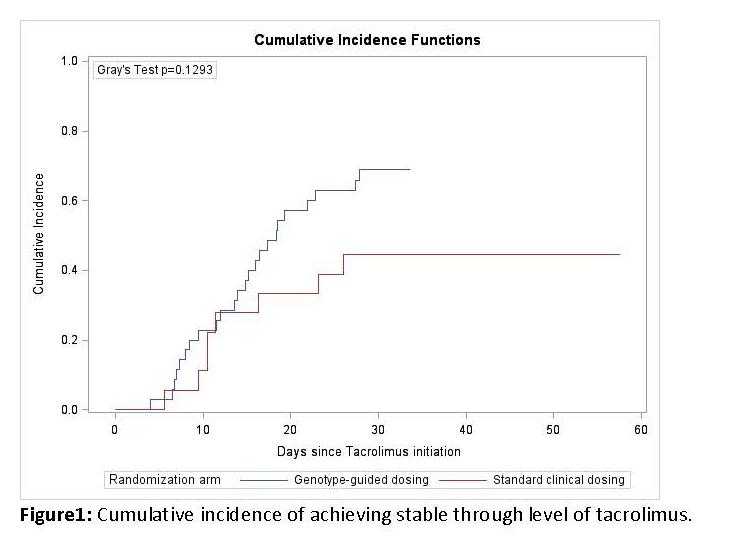 However, while 69% patients in the genotype arm had achieved stable therapeutic concentrations by 30-days, only 44% patients in the standard arm achieved stable therapeutic concentrations by 30-days (p=.089).
However, while 69% patients in the genotype arm had achieved stable therapeutic concentrations by 30-days, only 44% patients in the standard arm achieved stable therapeutic concentrations by 30-days (p=.089).
Conclusions: Age and genotype guided dosing in pediatric SOT was associated with fewer out of range tacrolimus levels and higher proportion of patients reaching stable therapeutic concentrations during 30 day follow up. This pilot trial highlights the importance of an individualized dosing algorithm for tacrolimus.
CITATION INFORMATION: Min S, Daljevic T, Lafreniere-Roula M, Manlhiot C, Nalli N, Grasemann H, Schwartz S, Kamath B, Ng V, Parekh R, Mital S. A Pilot Trial of Genotype-Guided Dosing versus Standard Clinical Dosing in Pediatric Solid Organ Transplants. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Min S, Daljevic T, Lafreniere-Roula M, Manlhiot C, Nalli N, Grasemann H, Schwartz S, Kamath B, Ng V, Parekh R, Mital S. A Pilot Trial of Genotype-Guided Dosing versus Standard Clinical Dosing in Pediatric Solid Organ Transplants. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/a-pilot-trial-of-genotype-guided-dosing-versus-standard-clinical-dosing-in-pediatric-solid-organ-transplants/. Accessed February 22, 2026.« Back to 2017 American Transplant Congress
