Applicability and Safety of a Regulatory T Cell Therapy in Adult Liver Transplantation: The “ThRIL” Phase I First-in-Human Trial.
MRC Centre for Transplantation, King's College London, London, United Kingdom
Meeting: 2017 American Transplant Congress
Abstract number: 200
Keywords: Adverse effects, Rapamycin, Rejection, Tolerance
Session Information
Session Name: Concurrent Session: Tolerance: Clinical Studies
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: E353C
Aim
“ThRIL” is a multiple site, open-label, uncontrolled, phase I clinical trial assessing the safety and applicability of a CD4+CD25+FOXP3+ regulatory T cell (Treg) immunotherapy in the setting of liver transplantation.
Methods
Adult recipients who have received a cadaveric liver allograft are treated with a single infusion of an in vitro expanded autologous polyclonal Treg product (TR002). Three sequential cohorts of 3 patients receive a low (0.5-1 million Tregs/kg), intermediate (3-4.5 million Tregs/kg) or high (5.0-6.5 million Tregs/Kg) dose. The first cohort of patients were recruited in the pre-transplant period, with procurement of whole blood immediately pre-transplant and infusion at 13 weeks post-transplant.
Limited applicability of the original trial protocol necessitated a protocol revision to recruit stable patients 6-12 months post-transplantation with leukapheresis utilised to procure the starting material and infusion at week 16 post enrolment [Figure 1]. Patients undergo a 6-month follow up period and a comprehensive immunomonitoring program.
Results
Over an initial 12-month recruitment period under the original trial protocol 62.5% of eligible patients were enrolled. 10% of patients were withdrawn for logistical reasons, 40% for receiving allografts outside of specified criteria and 30% for clinical reasons. 7 patients remained eligible at the time of transplantation. 4 patients were subsequently withdrawn prior to infusion due to post-operative complications, failure of dose manufacture or failure to meet progression criteria.
To date 3 patients have received the low dose with no dose limiting toxicities. Target enrolment has been achieved following protocol revision with dosing of cohorts 2 and 3 scheduled for January and April 2017 respectively.
Treg therapy appears to be safe in the setting of liver transplantation but has limited applicability when used in the early post-transplant period and a strategy targeting recipients at later time points may be more clinically relevant.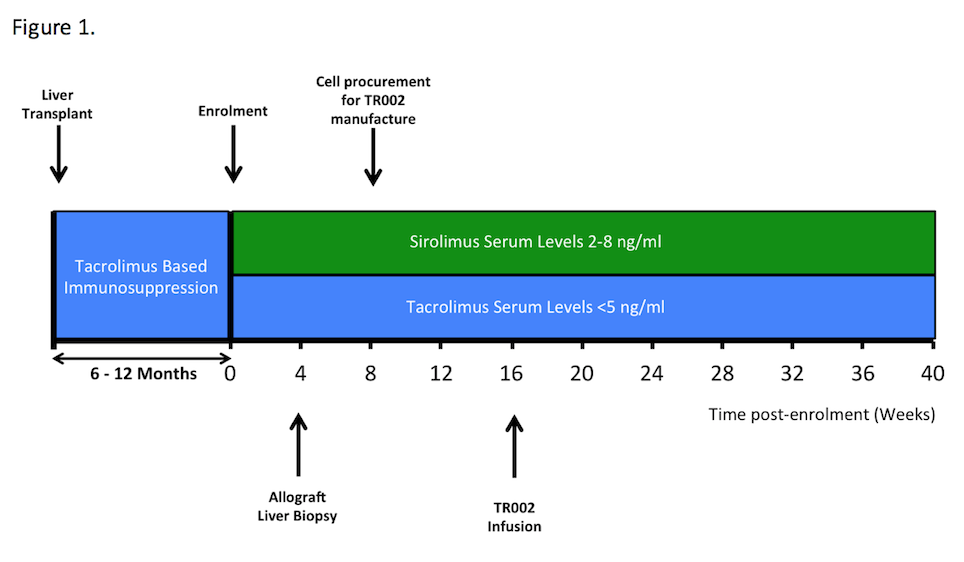
CITATION INFORMATION: Whitehouse G, Safinia N, Thirkell S, Fry L, Grageda N, Martinez-Llordella M, Lechler R, Heaton N, Lombardi G, Sanchez-Fueyo A. Applicability and Safety of a Regulatory T Cell Therapy in Adult Liver Transplantation: The “ThRIL” Phase I First-in-Human Trial. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Whitehouse G, Safinia N, Thirkell S, Fry L, Grageda N, Martinez-Llordella M, Lechler R, Heaton N, Lombardi G, Sanchez-Fueyo A. Applicability and Safety of a Regulatory T Cell Therapy in Adult Liver Transplantation: The “ThRIL” Phase I First-in-Human Trial. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/applicability-and-safety-of-a-regulatory-t-cell-therapy-in-adult-liver-transplantation-the-thril-phase-i-first-in-human-trial/. Accessed January 9, 2026.« Back to 2017 American Transplant Congress
