Genomic Analysis of Gene Expression in Human Kidney Transplants Identifies Moxd1 as a Key Driver for Renal Fibrosis.
1Division of Nephrology, Icahn School of Medicine at Mount Sinai, New York, NY
2Renal Section, James J Peter Veterans Affairs Medical Center, New York, NY
Meeting: 2017 American Transplant Congress
Abstract number: 186
Keywords: Fibrosis, Genomics, Kidney, Nephropathy
Session Information
Session Name: Concurrent Session: Pathways of Clinical Rejection
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: E351
Chronic allograft injury (CAI), characterized histologically by high chronic allograft dysfunction index (CADI) score on biopsy, is known to be a major determinant of graft loss after the first year of transplantation. In the GOCAR study we obtained serial biopsies (0, 3,12-months) to study differentially expressed genes that would associate with subsequent development of CAI. Our microarray data from surveillance biopsies demonstrated a strong correlation between Moxd1-expression at 3-months post-transplantation and a higher 12-month chronic allograft dysfunction index (CADI) score [figure1 A]. Since CKD and CAI are histologically similar, we examined transcriptional data from CKD models and observed significant up-regulation of MOXD1 in lupus nephritis, diabetic nephropathy and FSGS in human kidney tissues (Nephroseq data) and in murine ureteral obstruction (UUO) models. MOXD1 is a mono-oxygenase similar to DBH1, and MOXD1 gene showed multiple HIF-binding elements in the promoter region. To study whether MOXD1 expression was associated with hypoxia in allografts, we analyzed time-0 biopsies from allografts with/without delayed graft function (DGF). We observed Moxd1 expression was significantly increased in allografts with DGF vs those without[figure1 B]. In vitro, RNA sequencing showed that fibrosis related pathways were activated after Moxd1 over-expression in renal tubular epithelial cells[figure1 D]. In vivo, in UUO and aristolochic acid models, we found that Moxd1-knock out mice had significantly reduced pro-fibrosis marker transcripts[figure1 C], phosphorylation of Smad3[figure1 E], and renal fibrosis (measured by Picrosirius red, Masson, IF COL1A1)[ figure1 F], compared to wild type mice. These data suggest that Moxd1 is a novel marker in human allografts that may be initiated by ischemic injury and key mediator of subsequent renal fibrosis.
Please find the figures below. 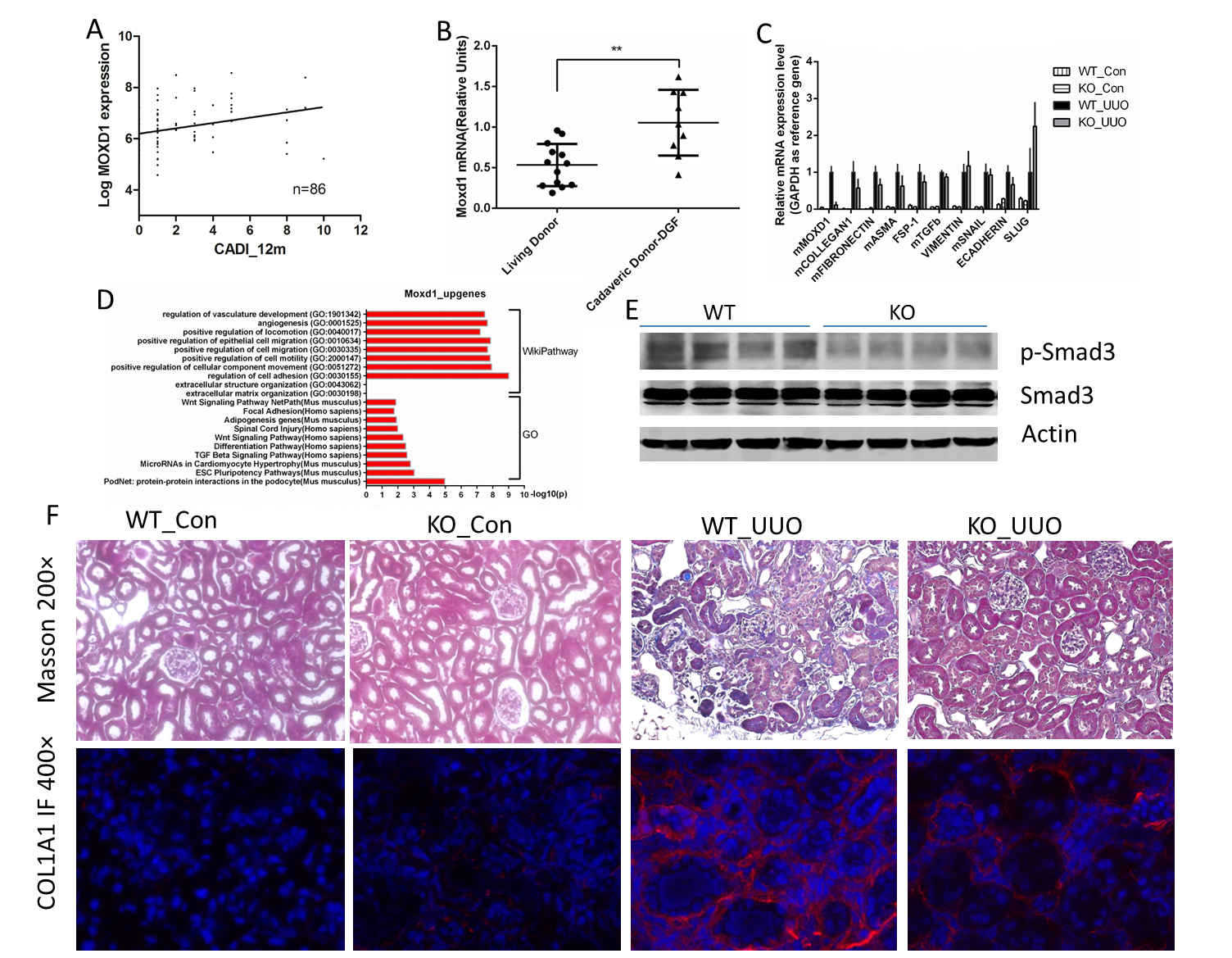
CITATION INFORMATION: Wei C, Menon M, Zhang W, Woytovich C, Philippe N, He J, Murphy B, Wei C. Genomic Analysis of Gene Expression in Human Kidney Transplants Identifies Moxd1 as a Key Driver for Renal Fibrosis. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Wei C, Menon M, Zhang W, Woytovich C, Philippe N, He J, Murphy B, Wei C. Genomic Analysis of Gene Expression in Human Kidney Transplants Identifies Moxd1 as a Key Driver for Renal Fibrosis. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/genomic-analysis-of-gene-expression-in-human-kidney-transplants-identifies-moxd1-as-a-key-driver-for-renal-fibrosis/. Accessed February 22, 2026.« Back to 2017 American Transplant Congress
