Abrogating Donor-Derived Myd88 Signaling Impairs the Recruitment of Recipient Neutrophils and CD8 T Cells into Lung Allografts in Mice.
1Comprehensive Transplant Center, Northwestern University Feinberg School of Medicine, Chicago, IL
2Division of Nephrology and Hypertension, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL.
Meeting: 2016 American Transplant Congress
Abstract number: 295
Keywords: Donors, Ischemia, knockout, Lung transplantation, marginal
Session Information
Session Name: Concurrent Session: Challenges to Graft Survival and Tolerance: Animal Models
Session Type: Concurrent Session
Date: Monday, June 13, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:30pm-5:42pm
Presentation Time: 5:30pm-5:42pm
Location: Room 306
Objectives: Transplant ischemia reperfusion injury (IRI) mediated primary graft dysfunction (PGD) remains the predominant cause of short- and long-term graft loss in lung transplantation. This study was designed to determine the role of donor-derived MyD88 signaling pathway in the development of PGD using a mouse model of lung transplantation.
Methods: Lungs from wildtype (wt) or MyD88 knockout (MyD88-/-) mice on BALB/c (H-2d) background were orthotopically transplanted into C57BL/6 (H-2b) mice. The recipients were euthanized at POD 1 and 5. Phenotypic analysis of graft infiltrating cells was performed using Flow-Cytometry. Additionally, graft histology was examined by H&E staining and graft function was evaluated by measuring arterial oxygenation (PaO2).
Results: Compared to normal controls, impaired lung function was observed in the wt allograft on POD 1, as manifested markedly decreased PaO2 level by 50%. In contrast, abrogation of donor MyD88 restored PaO2 level from 286.8±96.87mmHg to 614.7±31.2mmHg. Histology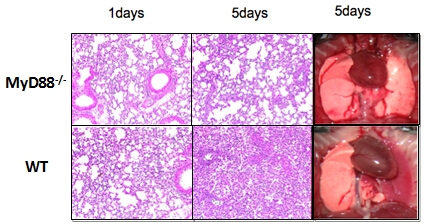 showed that there was a marked cellular infiltration in the wt allograft on POD 1 and the infiltration was further increased on POD 5, meanwhile the alveolar structure disappeared thoroughly. Parallelly to histology, phenotypic analysis of intragraft cellular infiltrates demonstrated neutrophil was the major intragraft infiltrating population in the wt allograft. Interestingly, MyD88 deficiency led to more than 50% decrease of neutrophil infiltration on POD 1 and further decreased on POD 5(p<0.01), and preservation of better lung integrity. Moreover, the recruitment of CD8+ T cell was reduced markedly as compare to the wt allograft on POD 5(p<0.05).
showed that there was a marked cellular infiltration in the wt allograft on POD 1 and the infiltration was further increased on POD 5, meanwhile the alveolar structure disappeared thoroughly. Parallelly to histology, phenotypic analysis of intragraft cellular infiltrates demonstrated neutrophil was the major intragraft infiltrating population in the wt allograft. Interestingly, MyD88 deficiency led to more than 50% decrease of neutrophil infiltration on POD 1 and further decreased on POD 5(p<0.01), and preservation of better lung integrity. Moreover, the recruitment of CD8+ T cell was reduced markedly as compare to the wt allograft on POD 5(p<0.05).
Conclusions: Abrogating donor-derived MyD88 signaling pathway restores graft function following lung transplantation. Targeting donor derived MyD88 signaling may be a potential therapy for PGD in clinical lung transplantation.
CITATION INFORMATION: Zheng Z, Wang J.-J, Yeap X, Kang X, Xie Y, Luo X, Zhang Z. Abrogating Donor-Derived Myd88 Signaling Impairs the Recruitment of Recipient Neutrophils and CD8 T Cells into Lung Allografts in Mice. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Zheng Z, Wang J-J, Yeap X, Kang X, Xie Y, Luo X, Zhang Z. Abrogating Donor-Derived Myd88 Signaling Impairs the Recruitment of Recipient Neutrophils and CD8 T Cells into Lung Allografts in Mice. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/abrogating-donor-derived-myd88-signaling-impairs-the-recruitment-of-recipient-neutrophils-and-cd8-t-cells-into-lung-allografts-in-mice/. Accessed February 11, 2026.« Back to 2016 American Transplant Congress
