HLA Antibody Clonality and Epitopes Dictate Complement Activation.
1Pathology and Laboratory Medicine, UCLA, Los Angeles, CA
2Leiden University Medical Center, Leiden, Netherlands.
Meeting: 2016 American Transplant Congress
Abstract number: A47
Keywords: B cells, Endothelial cells, Immunoglobulins (Ig), Polyclonal
Session Information
Session Type: Poster Session
Date: Saturday, June 11, 2016
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Halls C&D
Background: Alloimmunization results in production of polyclonal donor specific antibodies (DSA) against human leukocyte antigens (HLA), which mediate endothelial cell activation, leukocyte recruitment, and complement deposition, all hallmarks of antibody mediated rejection (AMR) in solid organ transplant. Bead-based assays allow for sensitive and specific detection of DSA. Recent additions to these assays using complement attempt to stratify patients more susceptible to rejection due to DSA complement-mediated pathogenicity. Despite these advances, graft loss due to chronic AMR remains a significant problem.
Aim: To dissect how DSA clonality and concentration, epitope location, and antigen density affect complement activation using a variety of clinically-relevant and customized platforms.
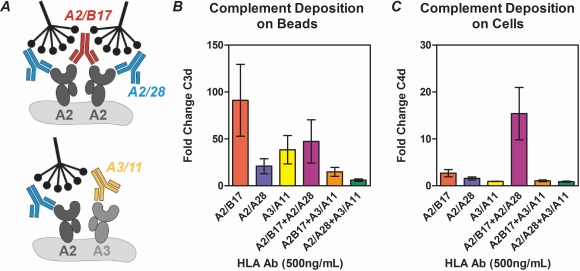
Methods/Results: We used monoclonal antibodies specific for different HLA molecules or different epitopes on the same HLA molecule to assess how concentration and clonality dictate complement activation (Figure A). Effects of antigen density on complement activation was compared using bead-based (C1q Screen, C3d Assay) and cell-based assays (CDC Assay, C4d deposition on human endothelium and B cells). We demonstrate (i) multiple DSA result in increased complement activation, (ii) antibodies present at low concentrations (<250 ng/mL) poorly activate complement in bead- and cell-based assays, (iii) antigen is more densely packed on beads than on cells, which negatively alters complement activation (Figure B), and (iv) complement activation by antibodies directed against different epitopes on the same HLA molecule is dependent on epitope location, and is significantly greater than that induced by a single antibody (Figure C).
Conclusions: These findings support eplet-matching for organ donation, as multiple antibody binding to different epitopes on the same HLA significantly increase complement activation. In this regard, the use of clinical assays in linkage (such as single antigen testing, C1q, and C3d) may provide a more accurate representation of the true complement-mediated pathogenicity of DSA.
CITATION INFORMATION: Thomas K, Valenzuela N, Mulder A, Reed E. HLA Antibody Clonality and Epitopes Dictate Complement Activation. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Thomas K, Valenzuela N, Mulder A, Reed E. HLA Antibody Clonality and Epitopes Dictate Complement Activation. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/hla-antibody-clonality-and-epitopes-dictate-complement-activation/. Accessed March 9, 2026.« Back to 2016 American Transplant Congress
