XR Tacrolimus Dosing By Ideal Body Weight Reduces Incidence Of Early Supratherapeutic Tacrolimus Concentrations
Pharmacy, NewYork-Presbyterian Hospital, New York, NY
Meeting: 2022 American Transplant Congress
Abstract number: 9089
Keywords: Dosage, Kidney transplantation, Weight
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
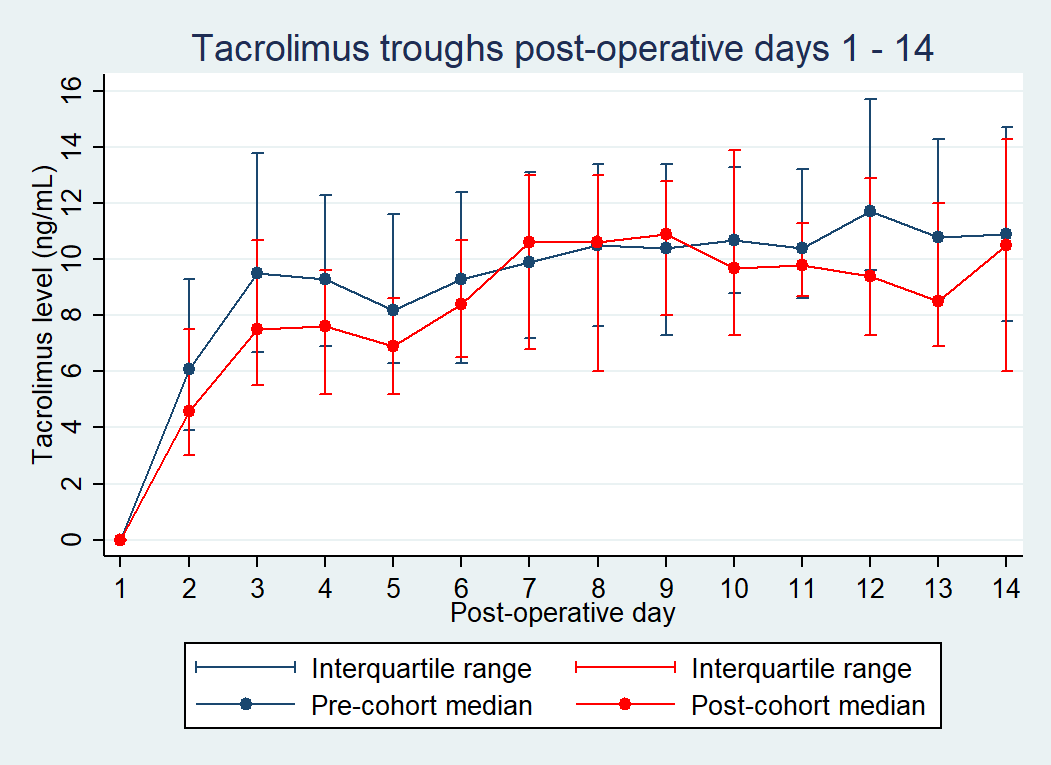
*Purpose: At our center, the use of de novo Envarsus XR® (extended-release [XR] tacrolimus) dosed at 0.12 mg/kg is standard of care in adult kidney transplant recipients (KTR). Based on a center-specific analysis, our center adopted a protocol change in weight-based dosing of XR tacrolimus from actual body weight (ABW) to ideal body weight (IBW). The purpose of this study was to compare the incidence of early supratherapeutic tacrolimus levels in the different weight-based cohorts.
*Methods: This was an IRB-approved, single center retrospective analysis of adults who received a KT between 07/2019-1/2021. Patients were excluded if they received medications that interact with tacrolimus or if they received different formulations of tacrolimus during the first 2 weeks post-KT. Due to associations between increasing age and increased risk of supratherapeutic tacrolimus levels, patients were matched 1:1 by age using nearest neighbor methodology. The primary endpoint was the difference in early supratherapeutic levels between the pre and post implementation of IBW dosing cohorts, defined as 1 appropriately timed tacrolimus trough greater than 12 ng/mL between postoperative day (POD) 2-4. Secondary endpoints included incidence of subtherapeutic levels < 6.0 ng/mL and < 4.0 ng/mL at any point between POD 5-14.
*Results: A total of 208 kidney transplant recipients (KTR) were identified. After 1:1 matching on age, 114 KTR were included in the analysis; 57 in each group respectively. KTR in the pre-cohort and post-cohort weighed 73.6 kg (IQR, 62.9 – 87.2) and 78.0 kg (IQR, 70.3 – 88.9) with IBW of 63.2 kg (IQR, 54.6 – 73.0) and 63.8 kg (IQR, 52.0 – 73.0), respectively. Median XR tacrolimus doses on POD1 were 9 mg (IQR, 8 – 10) in the pre-cohort and 8 mg (IQR, 7 – 9) in the post-cohort. Overall, the incidence of supratherapeutic concentrations was 42.1% in the pre-cohort vs. 19.3% in the post-cohort (p<0.008). Subtherapeutic levels were not statistically different between groups with the incidence of concentrations < 6.0 ng/mL observed in 40.4% vs. 43.9% (p=0.704) and < 4.0 ng/mL observed in 14.0% vs. 12.3% (p=0.782) of KTR, in the pre- and post-cohorts respectively. IBW dosing was associated with avoidance of early supratherapeutic tacrolimus concentrations aOR 0.3 (95%CI, 0.2 - 0.72; p=0.007).
*Conclusions: XR tacrolimus dosed empirically by IBW results in significantly less initial supratherapeutic levels with comparable subtherapeutic levels when compared with ABW dosing.
To cite this abstract in AMA style:
Codispodo G, Hedvat J, Salerno D, Lange N. XR Tacrolimus Dosing By Ideal Body Weight Reduces Incidence Of Early Supratherapeutic Tacrolimus Concentrations [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/xr-tacrolimus-dosing-by-ideal-body-weight-reduces-incidence-of-early-supratherapeutic-tacrolimus-concentrations/. Accessed July 15, 2025.« Back to 2022 American Transplant Congress