Waning Enthusiasm for a Once Promising Test: C1q Testing Does Not Provide Prognostic Information for DSA Screening Protocols
Medstar Georgetown Transplant Institute, Medstar Georgetown University Hospital, Washington, DC.
Meeting: 2018 American Transplant Congress
Abstract number: A113
Keywords: Antibodies, HLA antibodies, Kidney transplantation
Session Information
Session Name: Poster Session A: Kidney Acute Antibody Mediated Rejection
Session Type: Poster Session
Date: Saturday, June 2, 2018
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall 4EF
Introduction: Testing of donor specific antibodies (DSA) for binding of complement component C1q has been used to better elucidate pathologic antibodies from more benign reactions. However, results have been mixed and have not been looked at in DSA screening protocols.
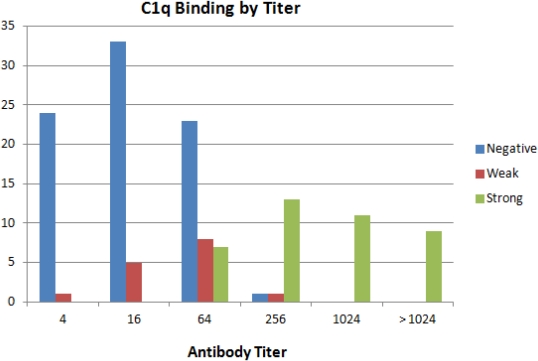
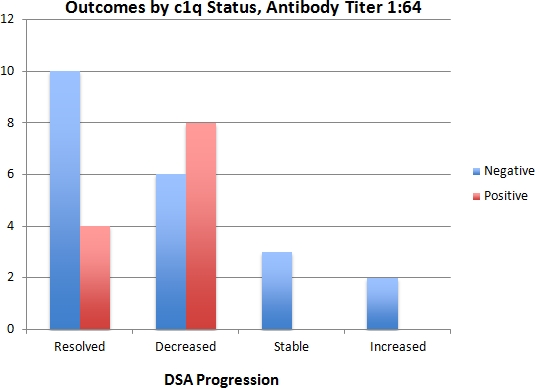
Methods: Our transplant center performs routine screening for DSA on all kidney transplant recipients. We retrospectively looked at all newly positive DSAs from the beginning of standard testing in 2014. C1q testing was scored as 'no binding', 'weak binding', or 'strong binding' based on MFI of the C1q assay. DSA strength was assessed by titer (1:4, 1:16, 1:64, 1:256, 1:1024, > 1:1024) at which antibody reactivity disappeared. In assessing for outcomes, antibodies were classified by the future trend of their titers as 'resolved' , 'decreased' , 'stable', or 'increased' .
Results: Since January 2014 there were 137 DSAs which were positive and tested for c1q. Most were treated before clinical sequelae and only 2 patients had biopsy proven AMR. Of the samples, 82 (60%) were C1q negative and 55 (40%) were positive. For antibodies with titers less than 1:64, 90% (57/63) of samples were c1q negative and none showed strong C1q binding. For antibodies with titers greater than 1:64, 97% (34/35) were positive with all but one showing strong binding. Only the 33 patients with a titer of 1:64 could be further stratified by C1q (21 negative, 12 positive) but among these, C1q was of no predictive value regarding resolution or worsening of the antibody.
Conclusions: When used in a screening protocol, C1q testing of DSA provided no additional predictive power beyond more traditional measures of antibody strength such as antibody titer.
CITATION INFORMATION: Gilbert A., Li D., Awwad M., Rosen-Bronson S., Abrams P., Verbesey J., Vranic G., Cooper M. Waning Enthusiasm for a Once Promising Test: C1q Testing Does Not Provide Prognostic Information for DSA Screening Protocols Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Gilbert A, Li D, Awwad M, Rosen-Bronson S, Abrams P, Verbesey J, Vranic G, Cooper M. Waning Enthusiasm for a Once Promising Test: C1q Testing Does Not Provide Prognostic Information for DSA Screening Protocols [abstract]. https://atcmeetingabstracts.com/abstract/waning-enthusiasm-for-a-once-promising-test-c1q-testing-does-not-provide-prognostic-information-for-dsa-screening-protocols/. Accessed February 28, 2026.« Back to 2018 American Transplant Congress