Varicella Hepatitis Mimicking Immune Checkpoint Inhibitor-induced Liver Toxicity
1Gastroenterology and Hepatology, University of Wisconsin, Madison, WI, 2Pathology, University of Wisconsin, Madison, WI
Meeting: 2021 American Transplant Congress
Abstract number: 1099
Keywords: Adverse effects, Hepatitis, Immunosuppression, Liver
Topic: Clinical Science » Liver » Liver: Immunosuppression and Rejection
Session Information
Session Name: Liver: Immunosuppression and Rejection
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Nivolumab is an anti-PD-1 monoclonal antibody belonging to a class of immune checkpoint inhibitors (ICI) used for many malignancies. A major limitation to their use is immune-related adverse events (irAEs) that lead to organ-specific inflammation that can be fatal if not promptly recognized and treated with aggressive immunosuppression. Immune-mediated acute liver injury (ALI) is a well described irAE and is often treated empirically. We describe a case highlighting the importance of early liver biopsy in patients not responding to treatment for immune checkpoint inhibitor-induced liver injury.
*Methods: N/A
*Results: A 70 year-old man with stage IIA esophageal squamous cell carcinoma status-post neoadjuvant chemo/radiation therapy and esophagectomy was treated with nivolumab. One month post-treatment he was admitted with likely nivolumab-induced myocarditis and was treated with methylprednisolone 1 mg/kg IV daily and one dose of infliximab. At discharge he was placed on an oral steroid taper. He had no liver injury during this stay.
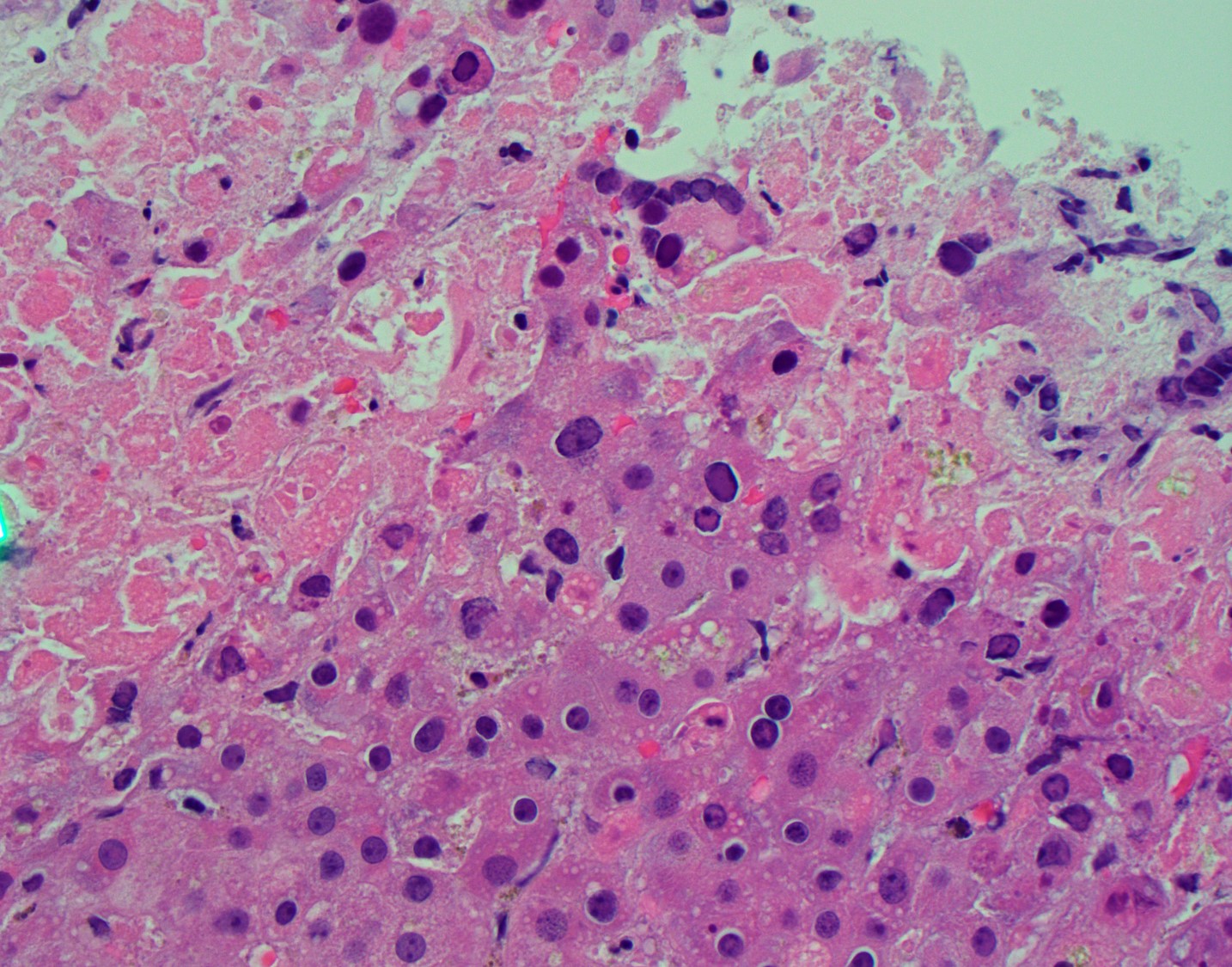
He returned within a week with abdominal pain and ALI with AST 2020 U/L, ALT 1750 U/L, bilirubin 3.2 mg/dL, alkaline phosphatase 211 U/L, INR 1.3. CT was unrevealing. He was treated with methylprednisolone and infliximab for suspected ICI-induced irAE. Thrombosis, autoimmune hepatitis, CMV and EBV were excluded. ALI worsened and hepatology was consulted (AST 3970 U/L, ALT 2883 U/L, alkaline phosphatase 203 I/L, bilirubin 5.4 mg/dL, INR 1.9). A liver biopsy was recommended, but he suffered cardiac arrest and expired the following day. Autopsy revealed disseminated varicella zoster virus (VZV) infection involving the liver (Fig. 1), lung, heart, bowel, esophagus and kidney.
*Conclusions: Recognition of immune-mediated liver injury has increased with the growing use of ICI, leading to protocoled immunosuppression regimens designed to empirically treat these often severe liver injuries. Unfortunately, these protocols are often initiated by oncology without hepatology involvement. Although this has simplified treatment and decreased the need for biopsy, it has removed hepatology from the care of patients with ALI. As this case highlights, patients receiving ICI are at risk of other forms of ALI, including drug-induced liver injury, cancer infiltration, infections and thrombosis. Hepatologists should advocate for complete evaluation of all patients with ALI. Biopsy should be considered early if they do not improve as expected.
Figure 1. Liver H & E. Areas of confluent necrosis with viral cytopathic effect.
To cite this abstract in AMA style:
Weiss MJ, Zhang X, Spengler EK. Varicella Hepatitis Mimicking Immune Checkpoint Inhibitor-induced Liver Toxicity [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/varicella-hepatitis-mimicking-immune-checkpoint-inhibitor-induced-liver-toxicity/. Accessed March 7, 2026.« Back to 2021 American Transplant Congress