Validation of Trugraf Gene Expression Profile in a Multicenter Cohort (TOGETHER Study)
1Mayo Clinic Jacksonville, Jacksonville, FL, 2Mayo, Rochester, MN, 3Mayo, Phoenix, AZ, 4Transplant Genomics, Framingham, MA
Meeting: 2022 American Transplant Congress
Abstract number: 1546
Keywords: Gene expression, Kidney transplantation
Topic: Basic Science » Basic Clinical Science » 17 - Biomarkers: Clinical Outcomes
Session Information
Session Name: Biomarkers: Clinical Outcomes
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The purpose of this study was to validate the performance characteristics of TruGraf GEP in a real-world population.
*Methods: This was a multicenter observational analysis of 4m and 12m surveillance biopsy-paired Trugraf results. Borderline rejection, TCMR, and ABMR were considered positive biopsies, while all biopsy findings other than subAR (including BK VAN, recurrent disease, IFTA) were considered negative. Copper-Pearson 95% CI were used for sensitivity, specificity, and accuracy; standard logit CIs were used for NPV and PPV. TruGraf microarray v1.3 with a 0.5 cutoff was used for results in order to reflect the commercially available product at that time.
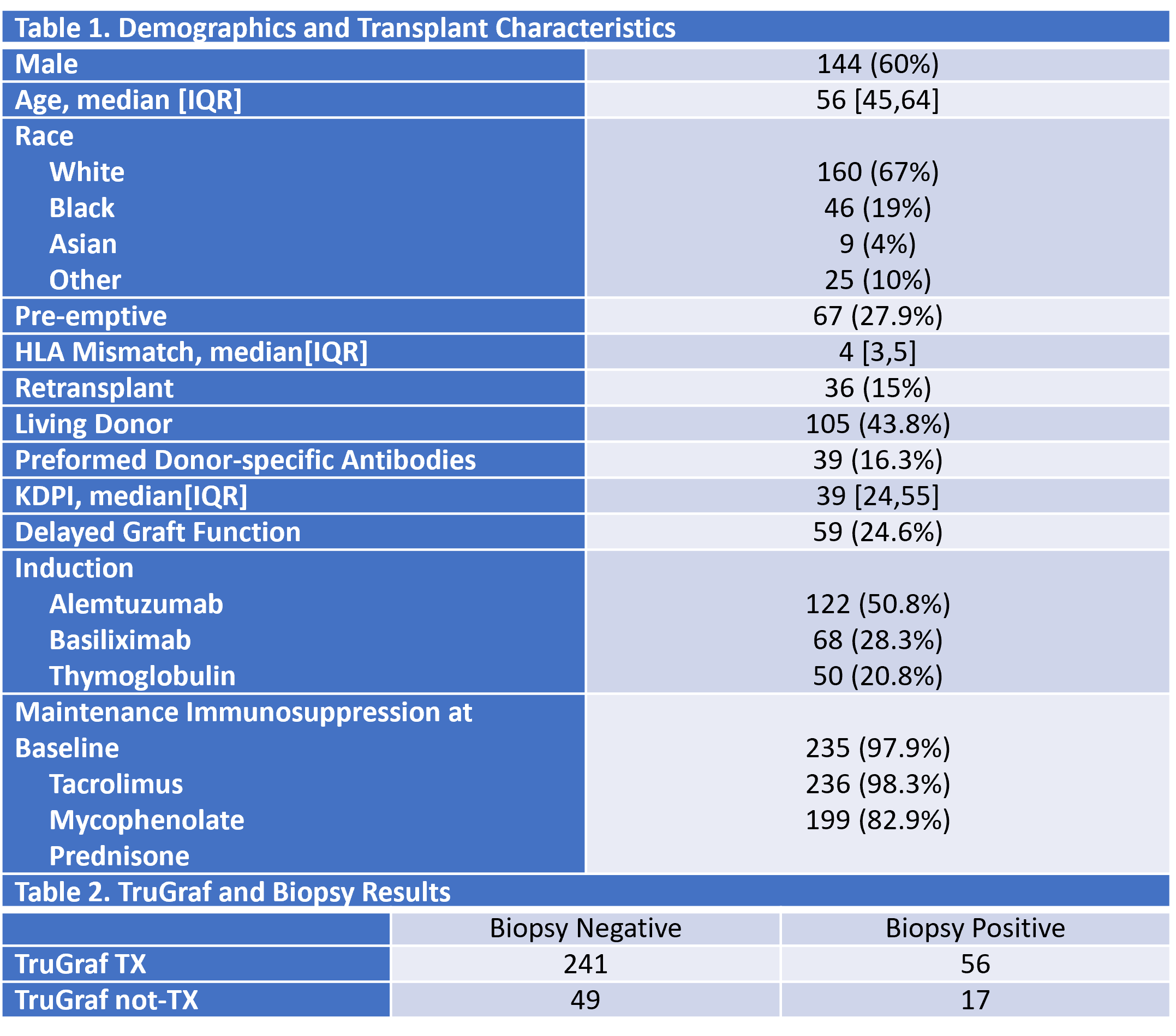
*Results: A total of 240 subjects were enrolled in the study, and 363 biopsy-paired TruGraf results were available. Demographics and transplant characteristics are in Table 1. Table 2 demonstrates the performance characteristics in the population. Sensitivity and specificity were 23.3% (14.2-34.7%) and 83.1% (78.3-87.2%), while NPV and PPV were 76.5% (73.9-78.8%) and 31.5% (22.0-42.8%) when adjusted to a 25% prevalence. Accuracy was 68.2% (63.1-72.9%). Prospective use of TruGraf could have eliminated 66% of surveillance biopsies accurately, while it would have been inaccurate in 29% of biopsies.
*Conclusions: The high NPV of TruGraf persisted in a real-world multicenter cohort, while the PPV was lower than validation studies (PPV 48%, 99 patients (Marsh, et al. Transplant Proceedings 2019). This is likely due to the inclusion of all comers and recent changes in Banff criteria. Further real world analysis of the combined biomarker panel OmniGraf is warranted.
To cite this abstract in AMA style:
Mai M, Park W, Heilman R, Smith B, First R, Fleming J, West-Thielke P, Holman J, Stegall M. Validation of Trugraf Gene Expression Profile in a Multicenter Cohort (TOGETHER Study) [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/validation-of-trugraf-gene-expression-profile-in-a-multicenter-cohort-together-study/. Accessed March 12, 2026.« Back to 2022 American Transplant Congress