Vaccine Responses in Combined Hematopoeitic Stem Cell-Kidney Transplant Recipients
1Northwestern University, Chicago, IL
2University of Louisville, Louisville, KY
3Regenerex, LLC, Louisville, KY.
Meeting: 2015 American Transplant Congress
Abstract number: C237
Keywords: Kidney transplantation, Stem cells, Vaccination
Session Information
Session Name: Poster Session C: Translational Biomarkers and Immune Monitoring
Session Type: Poster Session
Date: Monday, May 4, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Background: Vaccines remain one of the most effective methods of preventing infections following transplantation. Data suggests that vaccine responses are reduced in recipients of hematopoeitic stem cell (HSCT) or solid organ transplant (SOT) compared to healthy controls. There are no data on the efficacy of vaccines in combined HSCT-SOT recipients (R).
Methods: As part of an IRB approved study, chimeric vs. transiently chimeric recipients of combined HSCT-kidney transplant (KT) receive vaccination with TDaP, Pneumovax, HBV vaccine, polio, meningococcal, HiB and influenza vaccine consistent with current ASBMT guidelines. Revaccination data for HSCT-KTRs was retrospectively reviewed for tetanus, S. pneumonia and hepatitis B titers and compared between those who maintained and failed to have durable chimerism (DC). Descriptive statistics were assessed.
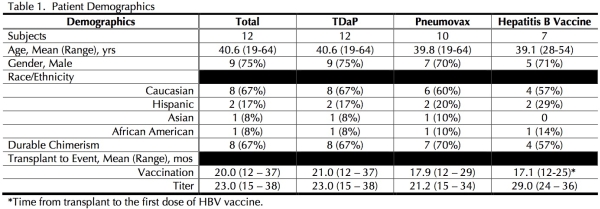
Results: A total of 12 combined HSCT-KTRs were vaccinated and had complete serologic follow-up (see Table 1). HSCT-KTRs with DC had higher rates of protective titers post-tetantus vaccination (see Figure 1). Likewise, HSCT-KTRs with DC demonstrated higher protective response rates in 12/14 serotypes following Pneumovax (see Figure 2). Rates of HBV seroconversion were higher among DC HSCT-KTRs: 3/4 subjects with DC did not have protective antibodies pre-vaccination; all patients had seroprotection post-vaccine. Only 1/3 of the patients without DC seroconverted (see Figure 3).
Conclusions: In this cohort of HSCT-KTRs, DC was associated with excellent seroprotection post-vaccination with TDaP, Pneumovax and HBV vaccine.
To cite this abstract in AMA style:
Feng C, Ison M, Galvin J, Ildstad S, Stare D, Leventhal J. Vaccine Responses in Combined Hematopoeitic Stem Cell-Kidney Transplant Recipients [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/vaccine-responses-in-combined-hematopoeitic-stem-cell-kidney-transplant-recipients/. Accessed February 17, 2026.« Back to 2015 American Transplant Congress
