Use of a Novel Bead-based Assay to Measure the Impact of Imlifidase on ABO IgG Antibodies
1Department of Pediatrics and Laboratory Medicine and Pathology, University of Alberta and Alberta Precision Laboratories, Edmonton, AB, Canada, 2Department of Pediatrics, University of Alberta, Edmonton, AB, Canada, 3Department of Chemistry, University of Alberta, Edmonton, AB, Canada, 4Hansa Biopharma, Lund, Sweden, 5Department of Pediatrics and Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada
Meeting: 2021 American Transplant Congress
Abstract number: 217
Keywords: Alloantibodies, Histocompatibility, Immunoglobulins (Ig), N/A
Topic: Clinical Science » Biomarkers, Immune Assessment and Clinical Outcomes
Session Information
Session Name: Biomarkers, Immune Assessment and Clinical Outcomes - III
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 7, 2021
Session Time: 4:30pm-5:30pm
 Presentation Time: 4:30pm-4:35pm
Presentation Time: 4:30pm-4:35pm
Location: Virtual
*Purpose: The accurate assessment and measurement of ABO antibodies (ABO-Ab) is a critical component of ABO-incompatible (ABOi) transplantation. ABO-Ab comprise both IgG and IgM, which are not readily distinguished by current hemagglutination (HA) methods and may not respond equally to Ab removal strategies. Imlifidase cleaves IgG, which prevents Fc-mediated effector functions. The majority of HA assays used clinically are not suitable for measuring the impact of imlifidase treatment since most are not Fc-specific and may include antibody-based reagents that can be cleaved by remaining imlifidase in the sample. Our aim was to assess the impact of imlifidase treatment using our novel bead-based assay to measure IgG and IgM ABO-Ab.
*Methods: Using Luminex single antigen beads, subtype-specific IgG and IgM ABO-Ab, expressed in mean fluorescence intensity (MFI), were measured in adult controls (n=75). ABO-Ab were compared before and after imlifidase treatment in healthy adult sera treated in vitro with imlifidase (n=8) and in sera from healthy volunteers treated with imlifidase, 0.25 mg/kg (18-HMedIdeS-15, n=11).
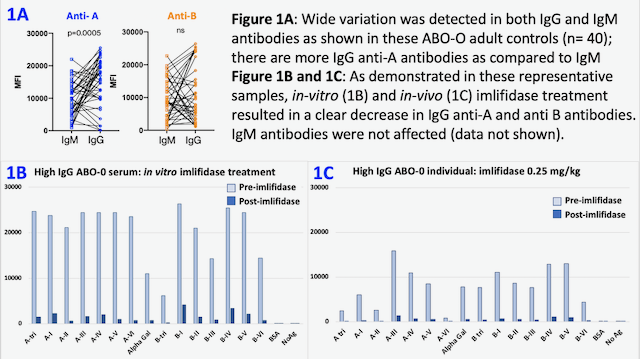
*Results: IgG and IgM ABO-Ab quantities vary widely in healthy individuals and include high MFI ABO-Ab (Fig 1A). In-vitro and in-vivo treatment with imlifidase effectively reduced MFI signal for IgG ABO-Ab (Fig 1B, 1C). IgM ABO-Ab MFI signal was unaffected.
*Conclusions: This novel bead-based assay enables measurement of both IgM and IgG ABO-Ab and facilitates evaluation of the role of isotypes in ABOi transplantation. ABO-Ab isotype differentiation may be particularly relevant in the context of plasmapheresis, commonly used in ABOi transplant, which more efficiently removes IgM Ab than IgG. HA has limitations in monitoring the effectiveness of imlifidase treatment but this bead-based antibody assessment adequately measures the effect of imlifidase and may provide a tool to clarify if there is a role for imlifidase in ABOi transplantation in individuals with high levels of ABO IgG Ab. Future studies will explore the use of a C1q-binding ABO assay to detect possible interference of single-cleaved IgG.
To cite this abstract in AMA style:
Halpin A, Motyka B, Ellis T, Pearcey J, Lowary TL, Runström A, Winstedt L, Bockermann R, Järnum S, Robertson A, West LJ. Use of a Novel Bead-based Assay to Measure the Impact of Imlifidase on ABO IgG Antibodies [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/use-of-a-novel-bead-based-assay-to-measure-the-impact-of-imlifidase-on-abo-igg-antibodies/. Accessed March 4, 2026.« Back to 2021 American Transplant Congress